Table of Contents
Overview
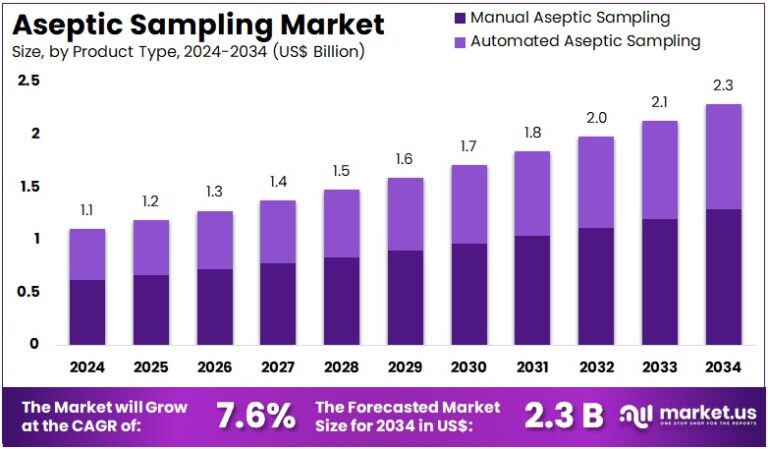
New York, NY – Aug 05, 2025 : The Global Aseptic Sampling Market is projected to reach around US$ 2.3 Billion by 2034. It is growing from US$ 1.1 Billion in 2024 at a CAGR of 7.6% during 2025 to 2034. North America led the market in 2024, capturing more than a 38.4% share. The region accounted for about US$ 0.4 Billion in value. This strong market presence is supported by advanced manufacturing capabilities and a high focus on quality standards across pharmaceutical and biotechnology companies.
The market is driven by the increasing demand for quality control in pharmaceutical and biopharmaceutical manufacturing. Aseptic sampling plays a vital role in preventing contamination during production. It ensures that critical processes remain sterile. Companies across the sector rely on these systems to maintain regulatory compliance and avoid product recalls. The focus on safer and more reliable drug production is directly fueling the market’s expansion. The need for consistent, contamination-free sampling is now more important than ever.
Real-time monitoring and accurate data collection are key growth factors in this market. As companies look to improve product quality and process efficiency, aseptic sampling tools are in higher demand. Sampling systems that provide instant feedback help in identifying issues early. This reduces batch failures and boosts operational reliability. Advanced aseptic techniques help manufacturers meet tight production timelines without compromising safety or sterility. As a result, demand continues to grow across both established and emerging markets.
Innovations are also helping shape the future of aseptic sampling. In January 2023, 908 Devices introduced the MAVEN device. It measures glucose and lactate levels during bioprocesses in real time. This enables better process control, enhanced precision, and improved safety in pharmaceutical production. Such advancements highlight the market’s shift toward smarter, data-driven tools. Devices like MAVEN support efficient bioprocess monitoring and align with the industry’s growing preference for automation and real-time insight.
The use of advanced aseptic sampling systems reduces contamination risks. It also helps optimize manufacturing operations by delivering more accurate and timely results. These improvements allow companies to enhance product yields and comply with evolving regulatory standards. With the biopharmaceutical sector expanding rapidly, the need for reliable and efficient aseptic sampling technologies will only rise. Opportunities for innovation and adoption of next-generation systems are growing. This makes the market a key area of interest for manufacturers and technology providers alike.
Key Takeaways
- In 2024, the aseptic sampling market earned US$ 1.1 billion and is projected to reach US$ 2.3 billion by 2034, growing steadily.
- The market is expanding at a 7.6% CAGR, indicating a rising demand for contamination-free sampling in pharmaceutical and biotech industries.
- Manual aseptic sampling dominated the product type segment in 2024, accounting for 56.3% of the total market share due to its widespread use.
- Among technologies, in-line sampling techniques took the lead, securing a 51.4% share thanks to real-time monitoring capabilities during production.
- Downstream processing was the top application in 2024, holding 58.5% of the market as it’s crucial for final product purity and testing.
- Pharmaceutical and biotechnology companies led end-user demand with a 60.2% market share, driven by strict quality standards in drug manufacturing.
- North America emerged as the leading regional market in 2024, capturing 38.4% of global revenue due to advanced infrastructure and high R&D investments.
Regional Analysis
North America Leads the Aseptic Sampling Market
North America holds the largest share of the aseptic sampling market, accounting for 38.4% of global revenue. This leadership is due to strict regulatory oversight. The US FDA continues to enforce Current Good Manufacturing Practices (CGMPs), emphasizing sterility and contamination prevention. Ongoing inspections and guidance stress the need for robust sampling systems. In Canada, Health Canada’s 2024 update to Annex 1 of its GMP guide further tightens standards for sterile drug production, supporting the demand for advanced aseptic sampling solutions across the region.
Asia Pacific to Register the Fastest Growth Rate
The Asia Pacific region is expected to witness the fastest CAGR during the forecast period. Regulatory authorities are raising their standards to align with global norms. China’s NMPA is strengthening pharmaceutical quality requirements with a focus on sterile procedures. In India, the CDSCO has introduced revised Schedule M and WHO TRS guidelines, effective August 2024. These rules stress stringent controls in sterile drug manufacturing. As a result, pharmaceutical firms are increasing their use of modern sampling tools to meet compliance and ensure product safety.
Segmentation Analysis
Product Type Analysis
In 2024, manual aseptic sampling led the market with a 56.3% share. Its dominance comes from strong use in pharmaceutical and food industries where contamination control is vital. This method offers flexibility and is cost-effective, making it ideal for settings with limited automation. Manual sampling also allows better control during collection. These factors make it a preferred choice in quality-sensitive environments. As demand for quality assurance grows in biotech and pharma production, the manual segment is expected to see steady expansion in the coming years.
Technology Analysis
The in-line sampling technique captured 51.4% of the market due to its efficiency and real-time capabilities. This method allows continuous monitoring without halting production, reducing downtime and improving accuracy. It is especially valuable in biopharmaceutical manufacturing, where sterility is critical. In-line sampling reduces contamination risks and enhances process control. As companies move toward automation and streamlined operations, adoption of this technology is set to rise. Its benefits in operational efficiency make it a key growth driver in aseptic sampling systems.
Application Analysis
Downstream processing accounted for 58.5% of the application segment in 2024. This stage includes purification and refining of biologics, which demand sterile and controlled environments. Aseptic sampling plays a vital role in ensuring product safety and consistency here. The growth of biologics and new therapies is boosting demand for sterile sampling methods. With rising regulatory pressure for clean and validated processes, companies are investing more in downstream technologies. This trend supports continued growth of aseptic sampling in critical post-production phases.
End-User Analysis
Pharmaceutical and biotechnology companies generated 60.2% of the total market revenue. Their reliance on aseptic sampling stems from strict regulations and the need for contamination-free manufacturing. These companies use sampling to ensure drug safety, especially in biologics and personalized medicine. With the pharma sector expanding globally, demand for sterile monitoring systems is growing fast. To meet regulatory and quality demands, more companies are adopting advanced sampling tools. This shift is expected to drive significant growth in this end-user segment over the forecast period.
Key Players Analysis
Key players in the aseptic sampling market use several strategies to boost growth. These include product innovation, acquisitions, partnerships, and global expansion. Companies aim to improve product functionality to meet rising demands in pharma, biotech, and food sectors. Many invest heavily in R&D to launch next-gen solutions that improve sampling accuracy and sterility. These steps help maintain a competitive edge in a fast-growing market. By focusing on efficiency and compliance, businesses ensure they meet industry standards and customer expectations.
In addition, companies are expanding into emerging markets to unlock new revenue opportunities. These regions offer high growth potential due to increasing pharmaceutical and bioprocessing activities. One notable player, Sartorius AG, is well-known for its lab equipment and solutions. The company focuses on the biopharmaceutical and life sciences industries. It provides advanced technologies that support sterile sampling and quality control. With its strong product portfolio and global reach, Sartorius continues to lead in offering reliable aseptic sampling solutions worldwide.
Emerging Trends
- Shift Toward Single-Use Sampling Devices: Many companies are now switching to single-use aseptic sampling tools. These disposable devices help reduce the risk of contamination during sampling. Since they are used only once, they remove the need for cleaning and sterilization between uses. This not only improves product safety but also saves time and lowers operational costs. In industries like pharmaceuticals and biotechnology, where hygiene is critical, single-use tools are becoming the preferred option. They also reduce downtime, support faster batch changes, and align well with current GMP standards. As a result, demand for disposable aseptic sampling devices is rising steadily across global manufacturing facilities.
- Growing Use of Automation: Automation is becoming more common in aseptic sampling systems. Manual sampling can lead to human errors, especially in high-volume production environments. Automated systems offer consistent results, better process control, and improved reliability. These systems are often used in pharmaceutical and biotech plants to meet strict quality and safety standards. Automation also saves labor time and reduces the risk of operator contamination. In large-scale operations, these benefits translate into better product quality and reduced operational costs. As production scales up, companies are turning to smart, automated sampling methods to stay efficient and compliant with global regulations.
- Stricter Regulatory Focus on Sterility: Regulatory agencies around the world are tightening rules on sterile drug production. Organizations like the US FDA and EMA now expect manufacturers to follow stricter GMP guidelines. This has led to more focus on contamination control and sterile practices in manufacturing. As a result, companies are investing in better aseptic sampling tools and methods. These solutions help ensure that products remain sterile during and after sampling. Compliance with regulations not only avoids penalties but also builds trust with customers. The push for higher sterility standards is a key driver behind innovation and adoption of advanced sampling technologies.
- Integration with Digital Monitoring Systems: Aseptic sampling devices are becoming smarter with digital integration. New systems can now track sampling time, location, and test results in real time. This improves traceability and helps meet documentation requirements for audits. Digital tools also support faster data analysis, which improves quality control. With better monitoring, it’s easier to detect and fix problems early in the process. Many modern production lines now require this type of connectivity. Integrating aseptic sampling with digital monitoring also supports Industry 4.0 goals. It helps manufacturers maintain high standards while keeping track of every step in the production cycle.
- Rising Demand from Biologics and Cell Therapy Industries: Biologics and cell therapies are becoming more popular in the medical field. These products need extremely high levels of sterility. Even small contamination can ruin a batch. As these sectors grow, the demand for advanced aseptic sampling solutions is increasing. Companies in these fields need tools that are precise, sterile, and easy to use. Sampling systems must also fit into cleanroom environments and support strict regulatory compliance. This has created a strong market opportunity for aseptic sampling device makers. The growth of biologics and cell therapy is pushing innovation in sampling technologies to meet rising needs.
- Sustainability and Eco-Friendly Design: Environmental concerns are shaping the design of aseptic sampling tools. Manufacturers are working to create systems that reduce plastic waste and use less energy. This is important because many single-use tools generate large amounts of waste. Eco-friendly designs aim to balance sterility with sustainability. Companies are also exploring recyclable materials and energy-efficient manufacturing methods. Customers are now more aware of their environmental impact and prefer greener solutions. Sustainable sampling tools help reduce the carbon footprint of pharmaceutical production. As climate regulations tighten, this trend is likely to grow stronger in the aseptic sampling market.
- Customized Sampling Solutions: More companies are asking for aseptic sampling tools that fit their specific needs. Off-the-shelf solutions don’t always work for complex or unique production lines. To solve this, manufacturers are offering modular and customized sampling systems. These can be tailored to match equipment design, process flow, and production scale. Custom tools also improve ease of use and reduce the chance of errors. This helps ensure accurate and safe sampling. The trend toward customization shows that one-size-fits-all solutions are no longer enough. As processes become more specialized, demand for tailored sampling devices is rising steadily.
Use Cases
- Pharmaceutical Manufacturing: In drug production, maintaining sterility is critical. Aseptic sampling helps pharmaceutical companies collect samples of liquids or powders without any contamination. These samples are taken during different stages of manufacturing. The goal is to check for harmful bacteria or foreign particles. It ensures the final product is clean, safe, and effective. By using aseptic techniques, companies avoid compromising large batches. This method also meets strict safety and quality regulations set by global authorities. As a result, aseptic sampling plays a vital role in the pharmaceutical industry’s quality control process. It helps reduce product recalls and improves patient safety.
- Biotechnology Labs: Biotech labs rely heavily on aseptic sampling to monitor living cultures. Scientists often grow cells or microorganisms to create vaccines, enzymes, or therapeutic proteins. Even a tiny contamination can ruin weeks of work. Aseptic sampling ensures that samples are collected without disturbing the sterile environment. It allows researchers to track the progress of cell growth and make adjustments in real time. This method improves efficiency and lowers the risk of failed batches. In biotechnology, accuracy and cleanliness are essential. Aseptic sampling provides both. It’s a trusted tool for monitoring and improving the production of sensitive biological materials.
- Food and Beverage Industry: In food and drink production, safety and hygiene are top priorities. Aseptic sampling is used to test milk, juices, and other beverages during processing. It helps detect harmful bacteria or microbes before the products are bottled or packed. This reduces the risk of contamination reaching consumers. Aseptic methods are especially important in high-speed production lines. They allow quick, sterile sample collection without slowing down the process. It also ensures compliance with food safety regulations. For companies, this means fewer recalls and higher customer trust. Aseptic sampling supports product quality, shelf life, and overall brand reputation.
- Water and Environmental Testing
In pharmaceutical and lab environments, water purity is non-negotiable. Aseptic sampling is used to collect water samples from purified systems. These samples are tested for microbial presence or contamination. This is important for water used in drug production, lab experiments, and cleaning systems. Even a small microbial imbalance can affect product quality or test results. Aseptic sampling ensures water systems remain clean and compliant with health standards. It helps facilities maintain Good Manufacturing Practices (GMP). Regular water testing keeps production safe and efficient. The process is simple but essential in any regulated environment. - Fermenttion Monitoring: Fermentation is used in industries like brewing, biofuels, and biopharmaceuticals. It involves growing microbes under controlled conditions. Aseptic sampling helps monitor these processes without disturbing the batch. Samples are taken to measure pH, temperature, and microbial activity. This ensures consistent product quality and reduces waste. In brewing, for example, aseptic sampling ensures the final product tastes right. In biotech, it’s used to produce compounds like antibiotics or vitamins. Keeping the process contamination-free is vital. Aseptic techniques allow quick testing while protecting the rest of the fermentation vessel. It’s a key quality assurance tool across multiple sectors.
- Cleanroom and Sterile Facility Monitoring: Cleanrooms are designed to stay free of particles and germs. Aseptic sampling helps verify that the air, surfaces, and equipment meet these strict standards. It’s commonly used in pharmaceutical plants, labs, and electronics manufacturing. Samples can be taken from the air or contact surfaces. This ensures that the sterile environment is not compromised. Regular aseptic monitoring helps detect early signs of contamination. It also supports compliance with regulatory standards. Without this, even minor exposure can ruin sterile batches or sensitive equipment. Aseptic sampling helps maintain safety, protect investments, and ensure top-quality production.
- Research and Development (R&D): During early drug research, scientists need sterile conditions. Aseptic sampling allows them to test new compounds without contamination. These samples are collected in small quantities to monitor reactions and study behavior. It’s important for tracking stability, purity, and safety. The process also supports innovation in gene therapy, cell therapy, and biopharmaceuticals. Aseptic sampling ensures the accuracy of data and integrity of the experiment. It minimizes the risk of external factors influencing test results. In R&D, precision and cleanliness go hand in hand. This technique is a trusted part of the drug development pipeline.
Conclusion
In conclusion, the aseptic sampling market is growing steadily due to rising demand for contamination-free production in pharmaceuticals, biotechnology, and food industries. Companies are focusing on quality control and sterility to meet strict global regulations. The shift toward automated, single-use, and digital sampling systems is helping improve safety, efficiency, and compliance.
Innovation, especially in biologics and cleanroom environments, continues to drive adoption. As manufacturing becomes more advanced and regulations become stricter, the need for reliable aseptic sampling tools will increase. This makes the market a strong area of opportunity for both established players and new entrants looking to support safer, smarter production practices worldwide.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



