Introduction
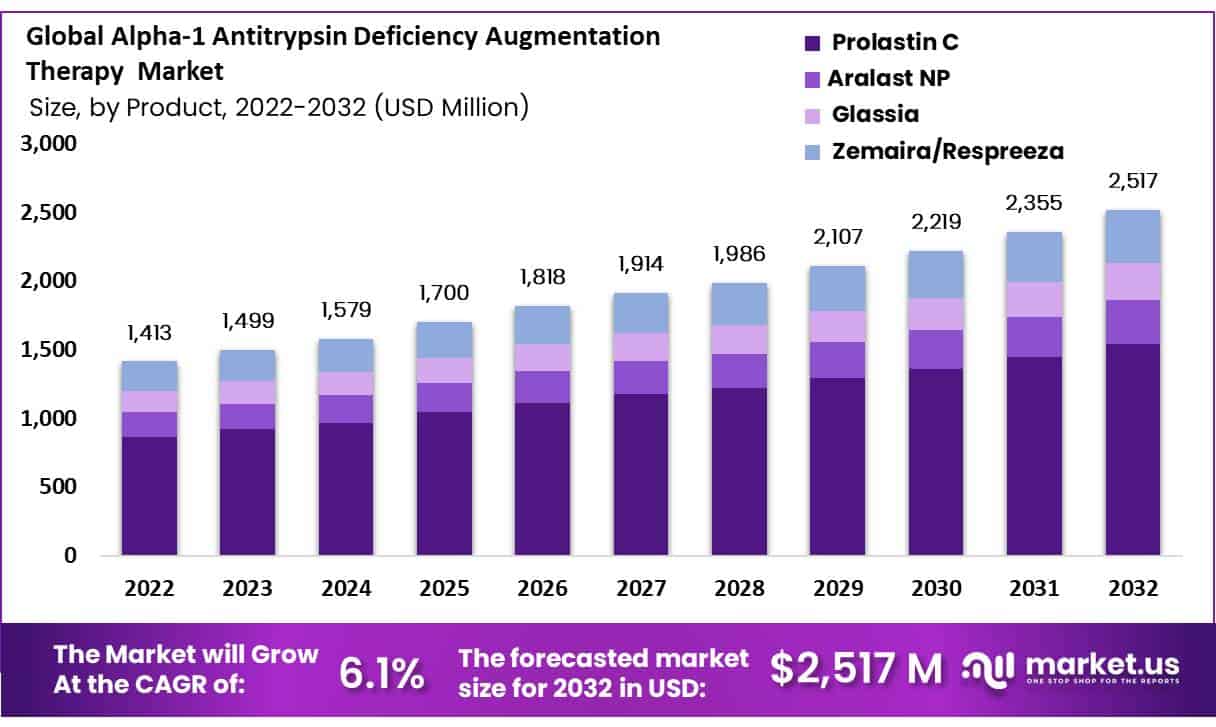
The Alpha-1 Antitrypsin Deficiency Augmentation Therapy Market is projected to grow from USD 1,413 million in 2022 to USD 2,517 million by 2032, advancing at a CAGR of 6.1%. This growth is driven by innovative treatment advancements and regulatory efforts aimed at enhancing patient outcomes. Emerging therapies, including gene therapy and non-plasma-based augmentation, alongside the adoption of surrogate endpoints like CT densitometry for quicker clinical trials and approvals, are pivotal growth factors.
There is a growing emphasis on employing surrogate endpoints, allowing for the rapid assessment of new treatments. This approach could expedite the introduction of novel therapies into the market, addressing both lung and liver complications associated with AATD. The shift towards these innovative and personalized treatment options is underpinned by ongoing research and investment in the field.
Furthermore, the integration of pulmonology and hepatology expertise underscores a holistic approach to patient care, enhancing symptom management and overall patient quality of life. Supportive regulatory frameworks and partnerships with AATD-focused foundations are essential for nurturing innovation. These collaborations and a flexible regulatory landscape are key to accelerating the approval and availability of breakthrough treatments. Continued advocacy and investment are crucial for expanding treatment possibilities and improving outcomes for AATD patients.
Key Takeaways
- The global AATD Augmentation Therapy market is projected to reach USD 2,517 million by 2032, growing at a 6.1% CAGR.
- Advances in genetic disease diagnostics are enhancing AATD treatment capabilities.
- Prolastin C and Glassia are leading in AATD therapy, with Prolastin C holding the largest market share.
- Hospitals are the primary users of AATD therapy, often for treating genetic and respiratory disorders.
- The market is driven by the prevalence of genetic disorders, effectiveness of therapies, and advancements in diagnostics.
- A major restraint is that augmentation therapy is not curative and may cause vein damage if improperly administered.
- Opportunities are expanding as AAT from healthy donors is utilized and awareness, technology, and government support increase.
- Currently, over 80 AATD molecules are in clinical trials, indicating a strong focus on innovation in the market.
- North America leads the market, supported by high awareness levels, government backing, and advanced diagnostics.
- With a revenue of USD 579.5 million in 2022, North America continues to dominate, driven by disease prevalence and leading companies.
Regional Analysis
Based on regional analysis, the Alpha-1 Antitrypsin Deficiency (AATD) Augmentation Therapy Market spans several regions including North America, Latin America, Eastern and Western Europe, Asia Pacific, and the Middle East & Africa. North America leads with the largest market share. This dominance is supported by robust awareness programs in the U.S. and Canada, focusing on AATD diagnosis and treatment, which are expected to drive market growth in the foreseeable future.
North America’s leadership in the market is further reinforced by the high disease prevalence and the strategic presence of key market players in the region. In 2022, North America recorded significant revenue, amounting to US$ 579.5 million, and is projected to maintain its leading position in the coming years.
In contrast, the Asia Pacific region is poised for rapid growth, with a significant Compound Annual Growth Rate (CAGR) anticipated during the forecast period of 2023-2032. This growth is driven by increasing government initiatives aimed at controlling and preventing genetic disorders and advancements in diagnostic procedures for critical diseases. The region’s expanding population further fuels this market expansion.
Europe, along with Asia Pacific, is also a key player in the AATD market. These regions are expected to hold substantial market shares, thanks to similar factors influencing North America, including rising disease prevalence and strategic initiatives by leading companies. Together, these factors contribute to a dynamic and evolving AATD augmentation therapy market landscape.
Emerging Trends
- Slowing Progression of Lung Disease: Augmentation therapy significantly helps slow down the progression of emphysema in patients with severe AATD. By boosting the levels of Alpha-1 Antitrypsin (AAT) in the body, the treatment shields lung tissues from destructive enzymes like neutrophil elastase. This protective mechanism is crucial as it helps maintain lung function over time, offering a better quality of life for those affected.
- Improving Survival Rates: Research suggests a notable improvement in survival rates for patients undergoing augmentation therapy. For example, a particular study highlighted that patients treated with augmentation therapy showed a 5-year mortality rate of 26%, which is markedly lower than the 35% mortality rate observed in those who did not receive the therapy. This improvement underscores the potential life-extending benefits of the treatment.
- Economic Considerations: Despite its high annual cost—potentially reaching up to $150,000—augmentation therapy may be economically viable in the long term. It has been shown to reduce other healthcare costs by slowing disease progression and minimizing hospital visits for exacerbations. A study pointed out that although patients receiving augmentation therapy incurred higher annual medical costs (approximately $127,537), this could lead to better overall health outcomes and significant long-term cost savings compared to the much lower annual medical costs ($15,874) for those not receiving the treatment.
Use Cases
- Slowing Progression of Lung Disease: Augmentation therapy significantly helps slow down the progression of emphysema in patients with severe AATD. By boosting the levels of Alpha-1 Antitrypsin (AAT) in the body, the treatment shields lung tissues from destructive enzymes like neutrophil elastase. This protective mechanism is crucial as it helps maintain lung function over time, offering a better quality of life for those affected.
- Improving Survival Rates: Research suggests a notable improvement in survival rates for patients undergoing augmentation therapy. For example, a particular study highlighted that patients treated with augmentation therapy showed a 5-year mortality rate of 26%, which is markedly lower than the 35% mortality rate observed in those who did not receive the therapy. This improvement underscores the potential life-extending benefits of the treatment.
- Economic Considerations: Despite its high annual cost—potentially reaching up to $150,000—augmentation therapy may be economically viable in the long term. It has been shown to reduce other healthcare costs by slowing disease progression and minimizing hospital visits for exacerbations. A study pointed out that although patients receiving augmentation therapy incurred higher annual medical costs (approximately $127,537), this could lead to better overall health outcomes and significant long-term cost savings compared to the much lower annual medical costs ($15,874) for those not receiving the treatment.
Conclusion
In conclusion, the Alpha-1 Antitrypsin Deficiency Augmentation Therapy market is poised for significant growth, driven by advancements in treatment methods and enhanced diagnostic capabilities. With a focus on personalized and innovative therapies, such as gene therapy and non-plasma-based options, the market is addressing both lung and liver complications associated with AATD effectively. The adoption of surrogate endpoints is accelerating the approval processes, bringing new treatments to patients faster. As regional markets like North America continue to lead with strong support and awareness, and as emerging regions show rapid growth potential, the overall market landscape remains dynamic and promising. This progression promises better patient outcomes and broadens the scope for future therapeutic advancements.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



