Table of Contents
Introduction
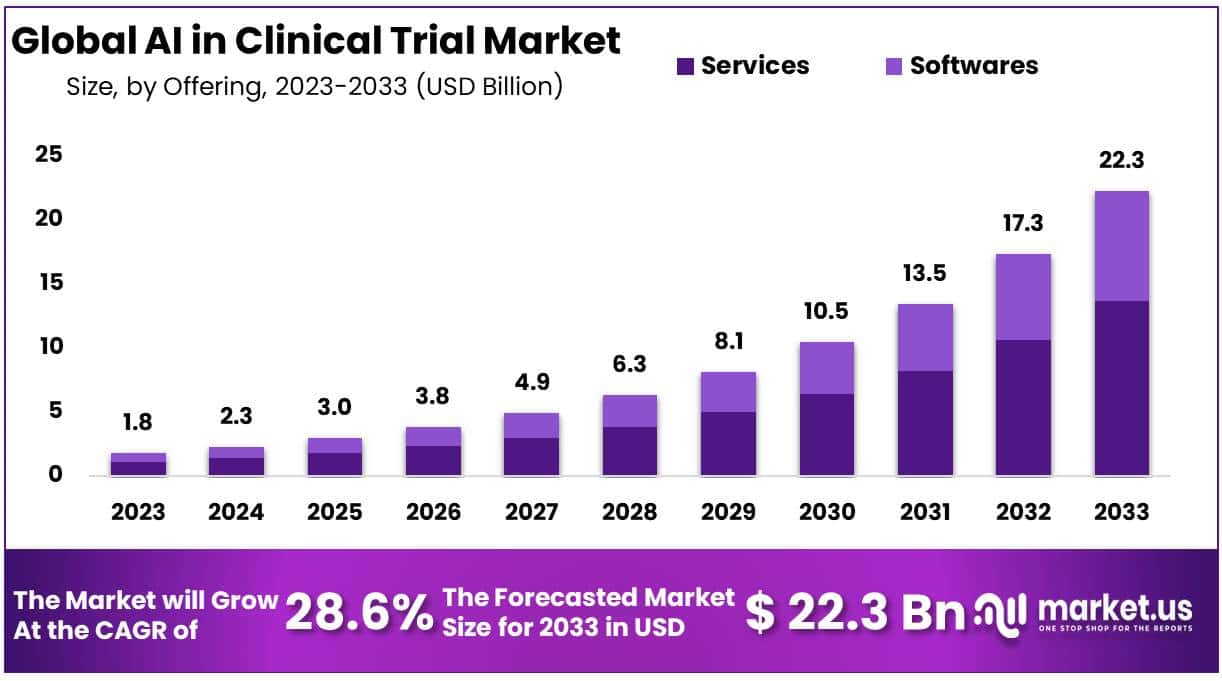
The AI in Clinical Trial Market is experiencing significant growth due to the rapid advancement and integration of artificial intelligence technologies within the clinical trials industry. In 2023, the market was worth USD 1.8 billion and is forecasted to reach USD 22.3 billion by 2033, with a CAGR of 28.6%. This growth is due to the increasing demand for innovative and efficient trial designs, especially with rising healthcare costs and the need for accelerated drug development timelines.
The market’s expansion is driven by several key factors. First, there’s a growing need to reduce the costs associated with clinical trials and shorten the time involved in the drug development process. This need aligns with the broader adoption of cloud-based applications and services in the industry. Additionally, AI’s role in enhancing the productivity and efficacy of clinical trials is crucial, as it improves decision-making processes at every stage – from asset and portfolio strategy to protocol and trial design. AI is used to identify promising indications for novel assets, refine trial eligibility criteria, optimize trial endpoints, and support portfolio strategy decisions, ultimately contributing to a more targeted and efficient clinical trial process.
However, the market does face some challenges, including the shortage of skilled AI workforce and the stringent regulatory guidelines that govern medical software. Despite these hurdles, the market is ripe with opportunities, particularly in the development of novel clinical trial designs for complex therapies and the burgeoning drug and biologics market. The integration of AI in clinical trials is proving instrumental in overcoming traditional limitations, such as the limited availability of datasets that hinder the scope and scale of clinical research.
Recent developments in the integration of artificial intelligence (AI) in clinical trials have highlighted significant advancements, notably through funding rounds and mergers and acquisitions (M&As) that underscore the sector’s growth and innovation. Unlearn.AI, a pioneering firm specializing in the development of digital twins for clinical trials, secured a substantial $50 million in a Series B funding round. This infusion of capital is aimed at enhancing the company’s capabilities in creating digital replicas of patients to facilitate smaller, more efficient studies, leveraging AI and historical data. Unlearn.AI’s approach represents a shift towards more nuanced and predictive modeling in clinical trial design, potentially reducing the duration and cost of trials.
Moreover, the clinical research organization (CRO) sector has witnessed a record number of M&As, with 50 completed deals being reported. This surge in M&A activity is indicative of a broader trend towards consolidation in the industry, as companies strive to expand their capabilities and adapt to the evolving landscape of clinical trials. The increase in M&A activity also reflects a strategic push to leverage new technologies and methodologies, including AI, to enhance the efficiency and effectiveness of clinical research.
These developments signify a growing recognition of the value of AI and digital technologies in transforming clinical trials, from patient recruitment and data analysis to trial monitoring and outcomes assessment. As investment continues to flow into AI applications for clinical trials, the potential for these technologies to streamline processes, reduce costs, and accelerate the development of new therapies becomes increasingly apparent.
Key Takeaways
- The AI In Clinical Trial Market was valued at USD 1.8 billion in 2023 and is forecasted to reach USD 22.3 billion by 2033, with a remarkable CAGR of 28.6%.
- Services segment dominated the market with a substantial market share of 61.3% in 2023, showcasing the importance of tailored solutions in meeting diverse clinical trial needs.
- Deep learning technology held a significant market share of 54.7% in 2023, highlighting its superiority in processing complex clinical trial data and improving diagnostics and patient stratification.
- The oncology application segment commanded a dominant market share of 45.9% in 2023, driven by the increasing prevalence of cancer globally and the development of AI tools tailored for oncology clinical trials.
- Pharmaceutical companies emerged as the leading end users, capturing a substantial market share of 65.8% in 2023, as AI facilitates efficient data analysis, accelerates drug development, and improves clinical trial outcomes.
- North America led the market with a notable market share of 31.5% in 2023, attributed to the presence of major market players, high adoption of AI technologies, and significant investments in healthcare innovation.
- The AI in Clinical Trial Market is anticipated to witness the fastest growth rate of 31.5% in the Asia-Pacific region, driven by increasing AI adoption, favorable government initiatives, and rising clinical trial enrollments.
- The market is characterized by strategic partnerships, product innovations, and mergers & acquisitions among key players such as Phesi, Intelligencia, DEEP LENS AI, and others.
AI in Clinical Trial Statistics
- Machine learning (ML) is used to find promising drug molecules and the right patients for clinical trials, leading to faster and smarter trial design.
- AI has improved how we choose trial sites and find patients, resulting in a 20.6% increase in patient enrollment in cancer studies.
- AI is making it easier to handle the huge amount of data in pharmacovigilance, with tools processing around 800,000 cases a year and automating 70% of the work.
- Clinical trial monitoring is more efficient with AI, reducing the time needed to solve queries by 37%, cutting down on data checking work by 31%, and speeding up trial close-out by 13 days.
- In patient care, AI helps find patients who might develop certain diseases like Alzheimer’s, with a success rate of 79% in identifying them correctly, much better than older methods.
- The journey from starting a clinical trial to getting a drug approved takes about 10 to 12 years, with only 12% of drugs making it through.
- Pharma companies use AI to try to improve these odds, aiming for better success rates in FDA approvals.
- The FDA has started to outline how AI and ML might be used in drug development as of May 2023, looking at how to keep the technology in check and ensure the data it uses is good quality.
- A Phase III clinical trial can generate over 3.56 million pieces of data, showing just how much information needs to be managed.
- Medical data is expected to grow by up to 5 times each year, highlighting the increasing role of AI in managing and making sense of this information.
Use Cases
Artificial Intelligence (AI) is significantly reshaping clinical trials, offering diverse applications that enhance efficiency, accuracy, and patient outcomes. Here are several key use cases illustrating AI’s transformative potential in clinical trials:
- Indication Selection for Asset Strategy: AI, combined with Real-World Data (RWD), aids in identifying promising indications for both existing and novel assets. This process involves analyzing patient outcomes under various conditions and leveraging data to expand or shift indication strategies effectively.
- Optimizing Protocol Design: AI’s role begins at the foundational stages of clinical trials by improving study protocols through historical data analysis. This leads to scientifically robust trials with efficient and patient-centric designs, significantly reducing amendments and enhancing the likelihood of success.
- Patient Recruitment: AI analyses patient data, EHRs, and medical literature to identify candidates who meet specific trial criteria, considering factors like location and demographic profiles. This method speeds up the recruitment process and ensures a more precise selection, addressing challenges such as patient accessibility to trial sites.
- Real-Time Safety Monitoring: Through continuous data analysis, AI identifies potential safety issues and adverse events in real-time, enabling prompt actions to safeguard patient safety and ensure regulatory compliance.
- Digital Twin Models: AI creates virtual replicas of patients based on genetic, medical history, and ongoing health data. These models simulate and predict outcomes, advancing personalized and safer healthcare solutions.
- Treatment Response Prediction: AI develops predictive models to assess patient responses to different treatments, optimizing efficiency and reducing risks. This approach is pivotal in personalizing medicine and improving patient outcomes.
Leveraging AI in clinical trials can improve treatment time-to-market, cost efficiency, regulatory compliance, enhanced data analysis and management, personalized medicine, and patient outcomes. AI reduces manual labor and repetitive tasks, allowing for a more streamlined and efficient drug development process.
Recent Developments
- Novartis and Chinook Therapeutics Deal: Novartis AG announced its plan to acquire Chinook Therapeutics for up to $3.5 billion. This move is part of Novartis’s strategy to focus on the renal therapeutics market, addressing the rising prevalence of chronic kidney disease.
- Roche’s Acquisition of Carmot Therapeutics: Roche entered into a definitive merger agreement with Carmot Therapeutics, planning to acquire the company and its clinical-stage obesity drugs portfolio for $2.7 billion, with an additional $400 million in potential milestone payments.
- Abbott’s Acquisition Plans for Bigfoot Biomedical: Abbott announced plans to acquire Bigfoot Biomedical in the third quarter of 2023, aiming to enhance its diabetes management portfolio.
- Eli Lilly to Acquire Dice Therapeutics: Eli Lilly disclosed plans to acquire Dice Therapeutics for $2.4 billion in cash, focusing on expanding its immunology capabilities.
- GSK’s Acquisition of Bellus Health: GSK reached an agreement to acquire Bellus Health Inc. for nearly $2 billion, aiming to access new treatments for respiratory diseases.
- Sanofi and Provention Bio Deal: Sanofi announced a $2.9 billion acquisition deal with US-based Provention Bio, aiming to enhance its portfolio in the healthcare sector.
- Pfizer’s Consideration for Seagen Acquisition: Pfizer is reportedly in talks to acquire cancer drugmaker Seagen Inc, following a previously considered acquisition by Merck that did not proceed.
- Envision Pharma Group Acquires OKRA.ai: Envision Pharma Group announced the acquisition of OKRA.ai, a leader in AI solutions for pharmaceutical operations. This acquisition aims to enhance Envision’s capabilities in delivering AI-driven insights for the pharmaceutical industry.
- AstraZeneca Buys CinCor Pharma: AstraZeneca announced its acquisition of CinCor Pharma in a deal valued at $1.8 billion, aiming to accelerate its development in treating cardiovascular diseases.
- Moderna’s First-Ever Acquisition of OriCiro: Moderna Inc acquired Japan-based OriCiro Genomics K.K. for $85 million, marking its first-ever acquisition. This deal aims to bolster Moderna’s biopharmaceutical applications.
Key Players Analysis
Phesi
Phesi is a notable player in the clinical trials sector, primarily through its AI-driven Trial Accelerator™ platform. This platform integrates vast amounts of real-world data from over 485,000 clinical trials and 60+ million patients to enable sophisticated scenario modeling and trial simulations. By providing a comprehensive view of patient data throughout the clinical development and commercialization process, Phesi helps life sciences companies to optimize study designs, thereby potentially saving them up to $7 million per phase III trial. The platform’s capability to simulate various trial outcomes and reduce unnecessary protocol amendments makes it a critical tool in enhancing the efficiency and success rates of clinical trials. Phesi’s innovative approach not only accelerates the development and commercialization of new drugs but also supports the creation of digital patient profiles and digital twins, which are essential for modern clinical trials.
Intelligencia
Intelligencia AI is carving a niche in the clinical trials sector by leveraging machine learning to enhance drug development outcomes. The company utilizes its AI-driven technologies to reduce risks and improve the predictability of clinical trials, ultimately aiming to make drug development faster and more efficient. Intelligencia’s AI models offer detailed insights into the success probabilities of clinical trials, relying on a comprehensive database of expertly curated data points.
Their flagship product, the Intelligencia Portfolio Optimizer™, integrates AI to provide on-demand insights that help pharmaceutical companies make informed decisions about asset management, portfolio strategy, and licensing opportunities. This technology is particularly praised for its ability to deliver unbiased and detailed analyses which are critical for high-stakes decision-making in drug development. By enhancing the precision of success predictions, Intelligencia AI supports companies in navigating the complexities of clinical trials, potentially leading to more effective therapies reaching the market sooner.
DEEP LENS AI
Deep Lens, a pioneering AI company, has significantly impacted the clinical trial sector by enhancing the recruitment process for cancer trials. Their platform, VIPER, integrates seamlessly into healthcare systems, combining lab results, EMR, and genomic data to identify the most suitable cancer patients for trials at the point of diagnosis. This process not only accelerates patient enrollment but also compresses the overall timeline for clinical studies, thereby facilitating faster market entry for new therapies.
Deep Lens’ collaboration with major oncology centers, such as the Norton Cancer Institute and New England Cancer Specialists, underscores its commitment to improving clinical trial efficiency. These partnerships aim to increase patient access to innovative treatments and streamline the complex logistics of trial recruitment. By automating the matching of patients to suitable trials, Deep Lens addresses the critical challenge of low patient participation rates in oncology research, which typically hover around three percent.
Halo Health Systems
Halo Health Systems is playing a significant role in the AI-driven clinical trials market. Launched in 2018, Halo Health Systems focuses on connecting patients remotely to clinical trials for new drugs and devices, leveraging IoT and mobile technology. This innovative approach helps to mitigate the need for in-person visits, enhancing patient engagement and compliance by sending reminders and updates about trial progress. Additionally, Halo Health Systems uses AI-driven search tools to match patients with suitable clinical trials based on their clinical data.
Pharmaseal
PHARMASEAL is significantly contributing to the AI-driven transformation in clinical trials. Established in 2016, this innovative company focuses on enhancing the efficiency and management of clinical trials globally through its comprehensive platform, Engility®. This platform integrates various management applications into a single solution, improving collaboration and governance across the clinical trial process. PHARMASEAL’s approach is particularly beneficial for small to medium-sized enterprises (SMEs) in the pharmaceutical and biotechnological sectors, providing them with cost-effective and scalable solutions.
The company has recently amplified its impact with a £1 million crowdfunding campaign aimed at expanding its technological offerings and supporting the commercialization of new products. This initiative reflects PHARMASEAL’s commitment to accelerating clinical trial processes and bringing new medicines to the market more swiftly. Their solutions have already gained traction, with notable adoption by a leading US health outcomes and technology company, underscoring the platform’s capacity to handle diverse trial needs while ensuring regulatory compliance and operational efficiency
Koneksa Health
Koneksa Health is making significant strides in the AI-driven digital biomarker space, particularly within the clinical trials sector. The company specializes in the development and application of digital biomarkers that are validated to rigorous FDA standards. These biomarkers enhance clinical trial efficiency by enabling remote data capture, which reduces patient burden and allows for the integration of data from various sources such as wearables and sensors. This method provides deeper insights into disease progression and patient responses to treatments.
Recently, Koneksa secured a $45 million Series C funding round led by AyurMaya with participation from notable investors such as Takeda Ventures, Merck, and Novartis. This investment underscores the industry’s recognition of Koneksa’s potential to transform clinical research methodologies. The funds are earmarked for expanding its digital biomarker platform and further developing its clinical data integration solutions, which are pivotal in supporting decision-making in drug development and enhancing patient-centric studies.
BioSymetrics
BioSymetrics is making significant strides in the AI-driven drug discovery sector, particularly in clinical trials. The company employs a unique phenomics-driven approach, integrating clinical and experimental data to enhance precision medicine initiatives. BioSymetrics has developed an innovative AI framework called Contingent AI™, designed to denoise and debias clinical data, which plays a crucial role in their phenotype-driven therapeutic discovery processes. Their AI capabilities enable them to harness massive datasets, including over 77 million longitudinal patient records and 1 million genomic profiles, to accelerate the discovery of effective drug targets and treatments.
Recently, BioSymetrics partnered with Sema4, utilizing their Elion platform and Sema4’s Centrellis® health intelligence platform. This collaboration aims to leverage AI and multi-omic data to initiate up to 10 therapeutic programs in areas such as cardiovascular, rare, and neurological diseases, focusing on diseases with high unmet medical needs
Google-Verify
Google’s involvement in the AI-driven clinical trial sector is primarily through Verily, its life sciences division. Verily focuses on developing AI tools and platforms that enhance the efficiency and effectiveness of clinical trials. The company’s technologies help bridge the gap between research and patient care by delivering data-driven insights and personalized health solutions. Verily collaborates with various healthcare and technology leaders to advance clinical research, exemplified by their partnership with GSK to form Galvani Bioelectronics, focusing on bioelectronic medicines.
In the broader AI in clinical trials market, services related to AI account for a significant share, indicating a robust integration of AI solutions in this field. AI technologies, particularly deep learning, are extensively used to optimize drug development processes and improve patient outcomes by providing more precise diagnostics and predictive analytics.
IBM Watson
IBM Watson has been instrumental in transforming clinical trial matching in the healthcare sector, particularly at the Mayo Clinic. Leveraging artificial intelligence, Watson has significantly improved the process of enrolling patients into clinical trials, particularly for diseases like breast cancer. Historically, only 5% of cancer patients participated in clinical trials due to the slow and manual process of matching patients to appropriate trials. With the integration of IBM Watson, the Mayo Clinic reported an 80% increase in enrollment in their breast cancer clinical trials. The AI system efficiently analyzes patient records and clinical trial criteria, enabling more accurate and quicker patient matches. This not only enhances patient access to potential treatments but also speeds up the advancement of medical research and the development of new therapies.
Symphony AI
Symphony AI is actively contributing to the AI-based clinical trials solution provider market, which is projected to grow significantly. Their focus is on leveraging AI to enhance the productivity and efficiency of clinical trials across various stages, thereby improving the cost-effectiveness and time efficiency of the drug development process. Symphony AI, along with other leading entities, is at the forefront of implementing AI technologies to optimize clinical outcomes, providing vital tools that assist pharmaceutical companies and research organizations in navigating the complexities of clinical trials.
BioAge Labs, Inc
BioAge Labs, Inc., a clinical-stage biotechnology company, is pioneering the application of AI in clinical trials, specifically in the development of treatments for muscle aging and other age-related diseases. Their AI-driven platform leverages proprietary human aging cohort data to identify key molecular pathways that influence healthy aging. One of their notable developments is the apelin receptor agonist BGE-105, which has shown promising results in Phase 1b trials. This drug has demonstrated significant improvements in muscle size, quality, and protein synthesis in elderly volunteers, particularly after periods of bed rest, which can severely impact muscle health in older adults.
Currently, BioAge is advancing BGE-105 into Phase 2 trials to explore its efficacy in preventing adverse outcomes in older patients, such as those under mechanical ventilation in intensive care units, a patient group with high unmet medical needs due to muscle atrophy and related conditions. This transition to later-stage trials underscores the potential of BioAge’s approach to address critical gaps in treatment options for aging-related health issues.
Ardigen
Ardigen, a key player in the integration of Artificial Intelligence (AI) into precision medicine, is notably active in enhancing clinical trial methodologies for immunotherapy. The company leverages AI to advance T-cell receptor (TCR) discovery, which is crucial for developing novel cell therapies. Ardigen’s technology significantly reduces the discovery and optimization time for TCRs, thereby decreasing the necessity for extensive laboratory experiments and lowering research and development costs. In September 2020, Ardigen received a substantial grant of $5.4 million to further develop its AI-driven TCR discovery platform, aiming to create a unique database of TCRs and their targets, as well as to produce and validate new TCR designs. This initiative not only exemplifies Ardigen’s innovative approach but also marks a significant contribution to accelerating global immunotherapy pipelines
Conclusion
These developments signify a growing recognition of the value of AI and digital technologies in transforming clinical trials, from patient recruitment and data analysis to trial monitoring and outcomes assessment. As investment continues to flow into AI applications for clinical trials, the potential for these technologies to streamline processes, reduce costs, and accelerate the development of new therapies becomes increasingly apparent.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



