Table of Contents
Overview
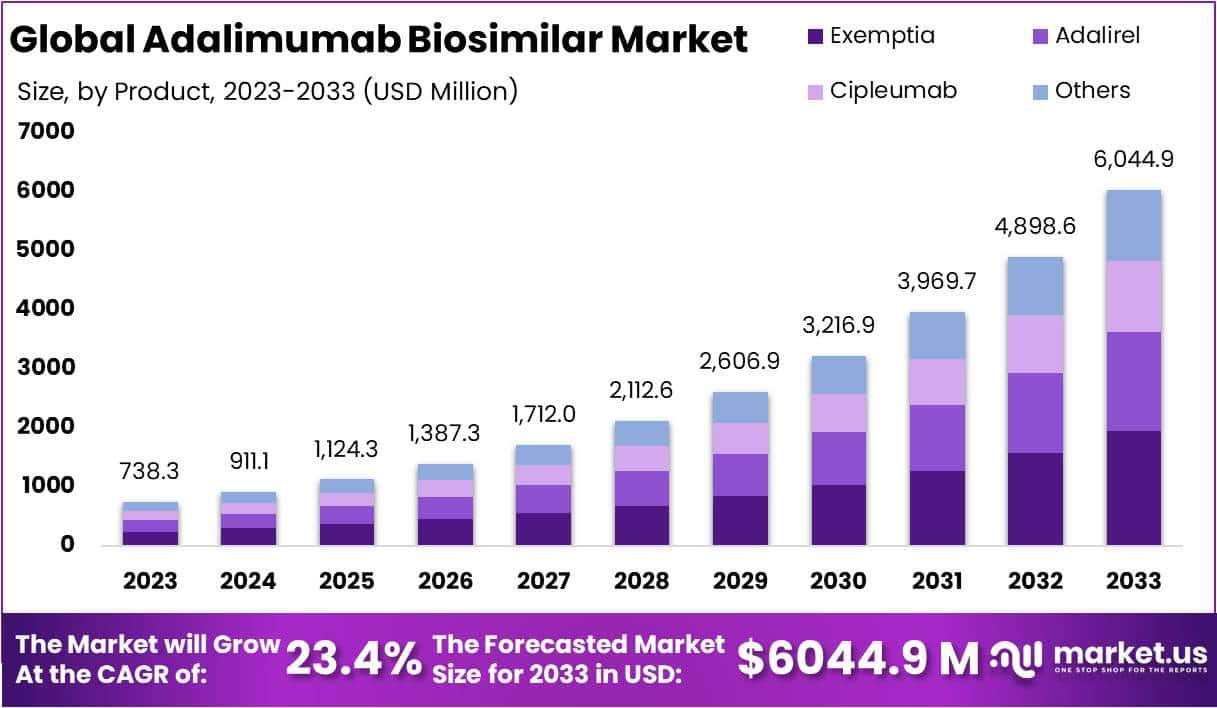
The Adalimumab Biosimilar Market is projected to grow from USD 738.3 million in 2023 to approximately USD 6,044.9 million by 2033, registering a strong CAGR of 23.4%. This growth is primarily driven by the expiry of Humira’s patent, which has opened the market to multiple biosimilar manufacturers. The resulting competition has improved accessibility and affordability. As healthcare systems increasingly adopt cost-effective biosimilars, both developed and emerging markets are witnessing significant uptake. The market’s trajectory reflects the growing emphasis on economical treatment options and the expanding biosimilar pipeline across therapeutic areas.
Regulatory bodies have established clear approval pathways and interchangeability guidelines for adalimumab biosimilars. These frameworks simplify substitution processes and build confidence among healthcare providers. Economic incentives such as favorable reimbursement policies and formulary inclusion have further strengthened market penetration. Governments and insurers are prioritizing biosimilars to reduce treatment costs and improve sustainability in healthcare budgets. This regulatory clarity, combined with payer support, has created a strong foundation for biosimilar adoption, particularly in hospital procurement and national tender programs aimed at reducing overall healthcare expenditure.
Robust clinical trials and real-world studies have confirmed that adalimumab biosimilars deliver comparable efficacy, safety, and immunogenicity to reference products. These findings have increased prescriber confidence and encouraged broader use across multiple disease areas. Continuous monitoring and data collection further reinforce safety profiles, supporting ongoing physician and patient trust. Healthcare institutions now view biosimilars as clinically reliable and economically beneficial. As trust deepens, switching from reference biologics to biosimilars is becoming a standard practice, fueling consistent market expansion across rheumatology, dermatology, and gastroenterology.
Innovation in formulation and delivery has enhanced the competitive edge of adalimumab biosimilars. New versions featuring high-concentration, citrate-free formulations and user-friendly autoinjectors improve patient comfort and compliance. These advancements make self-administration easier and reduce injection-site discomfort. Coupled with growing awareness initiatives among healthcare professionals and patients, such innovations have accelerated acceptance. Educational efforts continue to dispel misconceptions and highlight the safety and economic advantages of biosimilars, promoting their integration into routine clinical practice.
The future of the adalimumab biosimilar market remains promising, supported by a convergence of regulatory support, clinical validation, and payer endorsement. Expanding therapeutic indications and continuous innovation will further strengthen market performance. As healthcare systems prioritize affordability and sustainability, biosimilars are expected to play a central role in meeting these objectives. The combination of increasing patient access, reduced treatment costs, and global market expansion positions adalimumab biosimilars for sustained, long-term growth throughout the forecast period.
Key Takeaways
- The global Adalimumab Biosimilar market is projected to reach USD 6,044.9 million by 2033, expanding at a CAGR of 23.4% from 2024 to 2033.
- Exemptia led the product segment in 2023 with a 32.1% market share, supported by advanced formulation, proven efficacy, and competitive pricing strategies.
- Hospital pharmacies accounted for over 32.1% market share in 2023, benefiting from their strong presence and accessibility within healthcare institutions.
- Retail pharmacies demonstrated consistent growth, providing patients with convenient access to Adalimumab Biosimilar products across community and regional outlets.
- Market growth is primarily driven by rising chronic disease prevalence, patent expirations, cost-efficient healthcare demand, and favorable regulatory frameworks.
- Key challenges include complex manufacturing processes, stringent regulatory approvals, and low patient awareness regarding biosimilar alternatives.
- Prominent opportunities lie in emerging markets, strategic alliances, technological innovations, and expanded therapeutic indications for biosimilar use.
- Current trends feature biosimilar lifecycle management, patient assistance programs, unique branding approaches, and a shift toward self-administered formulations.
- North America dominated with 43.5% market share (USD 172.7 million) in 2023, supported by robust healthcare infrastructure and strong adoption rates.
- The Asia Pacific region is projected to witness the fastest growth, fueled by healthcare modernization, rising biologic demand, and favorable biosimilar adoption policies.
Regional Analysis
In 2023, North America dominated the adalimumab biosimilars market with a share of over 43.5%, valued at USD 172.7 million. The growth can be attributed to the region’s advanced healthcare infrastructure and supportive reimbursement framework. The presence of strong regulatory systems and healthcare awareness has encouraged early adoption of biosimilars. High disease prevalence, particularly autoimmune disorders such as rheumatoid arthritis and inflammatory bowel disease, continues to drive strong demand for adalimumab biosimilars across the region.
The United States has played a key role in this expansion due to the early entry and successful launches of adalimumab biosimilars such as Amjevita and Cyltezo. The high cost of branded adalimumab products has further accelerated biosimilar adoption. The presence of major pharmaceutical manufacturers and robust supply networks ensures accessibility and affordability. Furthermore, strong R&D capabilities and increasing collaborations between regional and global players have supported the biosimilars market’s steady development.
Europe held the second-largest share of the global market, estimated at around USD 112.6 million in 2023. The region benefits from favorable biosimilar regulations and well-established healthcare policies that encourage substitution. Patent expiries of branded biologics have allowed biosimilars to gain a stronger foothold. Government initiatives in countries like Germany, the United Kingdom, and France have promoted price competitiveness and expanded patient access. These factors collectively contribute to a sustainable and competitive European biosimilars market landscape.
Asia Pacific is projected to witness the fastest growth at a CAGR of approximately 27.4% from 2023 to 2030. The region’s large patient base, growing healthcare expenditure, and rising disposable incomes are key growth enablers. Emerging economies are developing biosimilar regulatory frameworks, improving access to affordable biologic therapies. Local and international manufacturers are expanding their production and clinical development capabilities. As governments strengthen approval pathways, the region presents significant long-term opportunities for biosimilar manufacturers and investors.
Segmentation Analysis
In 2023, the Exemptia segment dominated the Adalimumab Biosimilar market with a strong 32.1% share. Its success was driven by advanced formulation, proven efficacy, and affordability. Exemptia gained wide trust among healthcare professionals and patients due to its effectiveness in treating autoimmune diseases. Adalirel followed closely, supported by its quality and patient-focused results. Cipleumab, though newer, showed rapid growth and rising adoption. Together, these biosimilars strengthened market competition and expanded treatment options, reflecting growing acceptance of cost-effective biologic alternatives.
The Adalimumab Biosimilar market displayed a diverse distribution network in 2023. Hospital Pharmacies led with over 32.1% market share, benefiting from accessibility and integration within healthcare systems. Their proximity to doctors made them key for patient prescriptions and continuity of care. Retail Pharmacies also held a strong position, offering convenience and easy access for patients. Other channels, including specialty and online pharmacies, gained traction by serving niche needs. This evolving distribution landscape indicated innovation and adaptability in meeting patient demand efficiently.
Product
- Exemptia
- Adalirel
- Cipleumab
- Others
Distribution Channel
- Hospitals Pharmacies
- Retail Pharmacies
- Others
Key Players Analysis
The Adalimumab Biosimilar market is characterized by intense competition among leading players such as Amgen Inc., Boehringer Ingelheim, and Alfred E. Tiefenbacher. These companies are strengthening their positions through strategic expansions and collaborations. Significant investments are being made to enhance manufacturing capacities and ensure large-scale production efficiency. Continuous advancements in biosimilar technology have enabled these firms to achieve regulatory approvals across major regions. Their focus on maintaining high product quality and reducing production costs has been vital in addressing the rising global demand for affordable biologics.
Firms like Glenmark, Zydus Group, and Torrent Pharmaceuticals are actively investing in research and development. Their efforts are centered on innovation, clinical trials, and biosimilarity validation. By developing cost-effective formulations and expanding product portfolios, these companies aim to capture untapped market segments. Infrastructure enhancement, particularly in production and quality assurance, is also a priority. The continuous evolution of healthcare needs and the expiration of biologic patents have created significant opportunities for these manufacturers to strengthen their market presence.
Companies such as Emcure Pharmaceuticals, AET BioTech, and Coherus Biosciences are leveraging integration opportunities across the value chain. They are focusing on strategic alliances and licensing partnerships to access advanced technologies and streamline product distribution. These collaborations are enabling firms to accelerate commercialization timelines and expand their reach into emerging markets. Additionally, strong emphasis is placed on ensuring regulatory compliance and patient safety. Such initiatives have positioned these players as key contributors to the global biosimilar landscape.
Fujifilm Kyowa Kirin Biologics Co. Ltd. and other regional players are contributing to market growth through innovation-driven approaches. They are adopting advanced bioprocessing techniques to optimize yield and improve cost efficiency. Strategic market entry initiatives across Asia-Pacific, North America, and Europe are enhancing their competitive advantage. The overall market outlook remains positive, supported by continuous product launches and favorable healthcare policies promoting biosimilars. Collectively, these companies are shaping the future of the Adalimumab Biosimilar market through innovation, accessibility, and affordability.
Market Key Players
- Alfred E. Tiefenbacher
- Amgen Inc.
- Boehringer Ingelheim International GmbH
- Glenmark
- Zydus Group
- Torrent Pharmaceuticals Ltd.
- Emcure Pharmaceuticals Ltd
- AET BioTech
- Coherus Biosciences
- Fujifilm Kyowa Kirin Biologics Co. Ltd.
FAQ
Adalimumab Biosimilar FAQs
1. What is a biosimilar, and how is it different from a generic?
A biosimilar is a biological drug designed to be highly similar to an approved reference biologic. It has no significant differences in safety, purity, or effectiveness. Unlike generics, biosimilars are made from living organisms, so they cannot be exact chemical copies. They undergo strict testing to confirm similarity in clinical outcomes. Biosimilars help improve access to biologic treatments at lower costs while maintaining comparable quality and therapeutic performance as the original product.
2. What is the reference product for adalimumab biosimilars?
The reference product for adalimumab biosimilars is Humira, developed by AbbVie. Biosimilars of adalimumab are made to closely match Humira in structure, function, and clinical effects. These biosimilars treat autoimmune diseases like rheumatoid arthritis, psoriasis, and Crohn’s disease. Each biosimilar must demonstrate equivalent safety, efficacy, and immunogenicity in rigorous regulatory evaluations before approval. This ensures patients experience the same benefits and safety profile as they would with the original Humira therapy.
3. How many adalimumab biosimilars are approved or launched globally?
Multiple adalimumab biosimilars have been approved worldwide. More than ten have reached key markets such as the United States, Europe, and Asia. In the U.S., nine biosimilars entered the market in 2023 after Humira’s patent expiry. These include products from major companies like Amgen, Boehringer Ingelheim, and Sandoz. The growing list reflects strong competition and regulatory confidence in biosimilar development. This expansion is improving access and reducing treatment costs for patients with autoimmune disorders globally.
4. Are adalimumab biosimilars as safe and effective as the originator?
Yes. Adalimumab biosimilars must prove through comprehensive analytical and clinical studies that they are equally safe and effective as Humira. Regulatory agencies such as the FDA and EMA require robust comparability data. This includes testing for structure, biological activity, pharmacokinetics, and clinical outcomes. Post-marketing studies continue to confirm their reliability in real-world use. As a result, patients and healthcare providers can trust biosimilars to deliver consistent therapeutic benefits and comparable safety profiles.
5. Can patients switch from the originator adalimumab to a biosimilar?
Yes, most patients can safely switch from Humira to an adalimumab biosimilar under medical supervision. Clinical evidence shows no meaningful differences in safety, efficacy, or immunogenicity after switching. Regulatory agencies and healthcare organizations support such transitions to improve access and reduce healthcare costs. Some biosimilars are designated as interchangeable, allowing substitution at the pharmacy level. Regular monitoring ensures that patients maintain stable disease control and experience continued therapeutic effectiveness after the switch.
6. If a patient is switched and does not respond, can they revert to the originator?
Yes. If a patient does not respond adequately after switching to a biosimilar, they can revert to the original Humira. The decision should be guided by the treating physician based on clinical evaluation. In most cases, patients maintain similar responses after switching, but individual variability may occur. Close monitoring of disease activity, side effects, and treatment outcomes ensures optimal management. Physicians assess each case individually to ensure continuity of effective therapy and patient safety.
7. Does switching lead to immunogenicity or safety concerns?
Switching between Humira and biosimilars rarely leads to new safety or immunogenicity issues. Biosimilars must show similar immune response profiles to the originator in clinical studies. Regulators thoroughly review these results before approval. Real-world evidence and clinical trials confirm that most patients tolerate the switch well without increased antibody formation. Continuous pharmacovigilance programs track any rare reactions. Overall, switching is considered clinically safe and effective when done with appropriate medical supervision and patient education.
8. Are there differences in formulation among biosimilars and the originator?
Some adalimumab biosimilars differ slightly in formulation, such as concentration, injection volume, or excipients. Many are available in high-concentration, citrate-free versions that reduce injection discomfort. These formulation variations are reviewed and approved by regulatory agencies to ensure they do not affect safety or efficacy. Patients may notice improved convenience or comfort, but therapeutic outcomes remain consistent. Such innovations enhance patient experience while maintaining the same biological effectiveness as the reference Humira product.
9. Can a biosimilar be substituted automatically at the pharmacy?
Automatic substitution depends on national regulations. In the United States, only biosimilars with an “interchangeable” designation can be substituted at the pharmacy without prescriber approval. In Europe and other regions, substitution rules vary by country. Many health systems encourage biosimilar use but still require physician consent for changes. Educating patients and healthcare providers helps ensure smooth transitions. Regulatory clarity and transparent communication are key to building confidence and increasing adoption of biosimilar therapies.
10. What are the contraindications or precautions for adalimumab biosimilars?
Adalimumab biosimilars share the same contraindications as Humira. They should not be used in patients with active serious infections such as tuberculosis. Caution is advised in cases of heart failure, demyelinating disorders, or weakened immune systems. Patients should be screened for latent infections before starting treatment. Live vaccines are generally avoided during therapy. Regular monitoring for infection, allergic reactions, or liver issues is important. Physicians provide personalized guidance to ensure safe and effective treatment management.
Adalimumab Biosimilar Market FAQs
1. What is the size and growth rate of the adalimumab biosimilar market?
The global adalimumab biosimilar market is expanding rapidlyand Is estimated to reach 6044.9 Million by 2033. It is projected to grow at a compound annual growth rate (CAGR) of around 23.4% during 2025–2034. Growth is driven by rising autoimmune disease prevalence, patent expiry of Humira, and increasing biosimilar approvals. North America and Europe dominate the market, while Asia Pacific shows the fastest growth. The entry of multiple manufacturers is creating strong price competition and increasing accessibility for patients worldwide.
2. What are the key market drivers?
Several factors drive the adalimumab biosimilar market. Patent expiration of Humira has opened opportunities for biosimilar developers. Rising cases of rheumatoid arthritis, psoriasis, and Crohn’s disease increase demand. Governments and payers support cost-saving therapies to reduce healthcare spending. Advances in biomanufacturing improve quality and scalability. Growing awareness and acceptance among physicians and patients further accelerate adoption. Together, these drivers make biosimilars an attractive option for sustainable treatment access and healthcare system savings.
3. What are the main market restraints or challenges?
The market faces several challenges despite strong growth potential. High manufacturing costs, complex production processes, and stringent regulatory requirements limit entry for smaller players. Physician hesitation, patient concerns, and limited interchangeability policies slow adoption in some regions. Competitive pricing pressures reduce margins for companies. Additionally, dominance of the originator through rebates and brand loyalty affects market penetration. Overcoming these barriers requires education, transparent communication, and policy support to build greater confidence in biosimilars.
4. Which regions lead the adalimumab biosimilar market?
North America and Europe currently lead the adalimumab biosimilar market. These regions benefit from strong regulatory frameworks and established healthcare infrastructure. In the United States, multiple biosimilars launched in 2023, increasing competition. Europe remains a mature market due to early biosimilar adoption and supportive reimbursement systems. Asia Pacific is emerging as the fastest-growing region. India, China, and South Korea are developing cost-efficient manufacturing capabilities, creating export opportunities and boosting regional market expansion.
5. Who are the key players in the adalimumab biosimilar market?
Major companies in the adalimumab biosimilar market include Amgen, Boehringer Ingelheim, Sandoz, Samsung Bioepis, and Pfizer. Indian firms like Zydus Lifesciences, Glenmark, and Hetero also play significant roles. These companies produce biosimilars such as Amjevita, Cyltezo, Hyrimoz, and Exemptia. Their strategies include global partnerships, competitive pricing, and product differentiation through improved formulations. Continuous investment in biologic manufacturing and clinical research strengthens their market position and helps expand patient access worldwide.
6. How competitive is the biosimilar field relative to the originator?
Competition in the adalimumab biosimilar field is intense. After Humira’s patent expiry, multiple biosimilars entered the market, leading to aggressive pricing. However, AbbVie maintains a large market share through discounts, contracts, and brand loyalty. Biosimilar companies are differentiating products by offering high-concentration or citrate-free formulations. Increased payer support and insurance coverage are helping biosimilars gain traction. Over time, growing familiarity and cost benefits are expected to shift market balance toward biosimilars.
7. What has been the uptake behavior of adalimumab biosimilars in practice?
The adoption of adalimumab biosimilars started slowly but is now increasing steadily. In the United States, uptake was low initially due to rebate contracts and formulary restrictions. However, payer reforms in 2024 improved biosimilar access and boosted adoption. In Europe, switching rates have been higher due to favorable policies. Physicians and patients are gradually gaining confidence as clinical evidence grows. As awareness spreads, market share for biosimilars continues to rise across major regions.
8. What are recent trends or shifts in the market?
Recent trends show rapid market diversification and strategic partnerships. Many payers are now preferring biosimilars over Humira to reduce costs. Co-promotion agreements between innovators and biosimilar firms are emerging. Manufacturers are introducing improved formulations like high-concentration or pain-free injections. Digital education and patient support programs are increasing biosimilar acceptance. Global competition and tender-based pricing models are reshaping pricing structures. These shifts indicate a maturing market focused on cost efficiency and expanded patient access.
9. What is the role of insurance, reimbursement, and regulatory policy in market growth?
Insurance and reimbursement policies play a vital role in biosimilar market expansion. Favorable formulary placement, automatic substitution, and competitive pricing drive adoption. Governments and payers often promote biosimilars to control biologic drug costs. Regulatory frameworks defining interchangeability further support confidence in switching. In contrast, restrictive reimbursement or rebate agreements with originators can slow progress. Clear regulations, transparent pricing, and supportive healthcare policies are essential for sustainable biosimilar growth and market stability.
10. What is the future outlook for the adalimumab biosimilar market?
The adalimumab biosimilar market is expected to grow strongly over the next decade. Increasing product launches, improved formulations, and competitive pricing will expand patient access. Real-world data will enhance confidence in biosimilar safety and effectiveness. Emerging markets will contribute significantly to growth through local production. Challenges such as pricing pressure and complex regulations remain, but overall prospects are positive. With strong policy support, the market is positioned for sustained expansion and greater healthcare affordability.
Conclusion
The Adalimumab Biosimilar market is expected to grow strongly in the coming years due to patent expirations, rising autoimmune diseases, and strong regulatory support. Increased trust among doctors and patients, along with cost-effective treatment options, is driving widespread adoption. Continuous innovation in formulations and delivery methods is improving patient comfort and convenience. Supportive reimbursement policies and government initiatives are further boosting market penetration. As healthcare systems focus on affordability and access, biosimilars will play a key role in reducing treatment costs and improving patient care. The market outlook remains positive, supported by expanding global demand and continuous product advancements.
View More
Biosensors Market | Biosimulation Market | Infection Control and Biosafety Products Market | Biosecurity Market | Biosimilars Market
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



