Introduction
The Global Adalimumab Biosimilar Market Size is projected to grow from USD 738.3 million in 2023 to approximately USD 6,044.9 million by 2033, reflecting a robust compound annual growth rate (CAGR) of 23.4%. This significant expansion is driven by patent expirations, cost-effective alternatives, regulatory approvals, and stakeholder acceptance, paving the way for increased accessibility to these biologics.
The expiration of patents for AbbVie’s Humira has created opportunities for biosimilar manufacturers to enter the market. Products like Sandoz’s Hyrimoz have introduced affordable alternatives, spurring competition and driving market growth. Regulatory approvals have bolstered market entry, with multiple biosimilars now available. Despite this, AbbVie’s Humira retained over 80% of the U.S. market share due to strategic pricing and pharmacy benefit manager dynamics, showcasing the complexities of market penetration.
Biosimilars significantly reduce healthcare costs. For instance, the UK’s National Health Service projected savings of £300 million by transitioning to adalimumab biosimilars. Educational initiatives highlighting the safety and efficacy of biosimilars have improved acceptance among healthcare providers and patients. These efforts are essential for fostering confidence and driving adoption in the broader healthcare community.
Strategic collaborations have enhanced biosimilar uptake. Partnerships like CVS Health’s Cordavis working with Sandoz to promote Hyrimoz have streamlined formulary changes and boosted prescriptions. Such alliances underline the importance of collaborative efforts in accelerating market growth and ensuring broader access to affordable treatment options.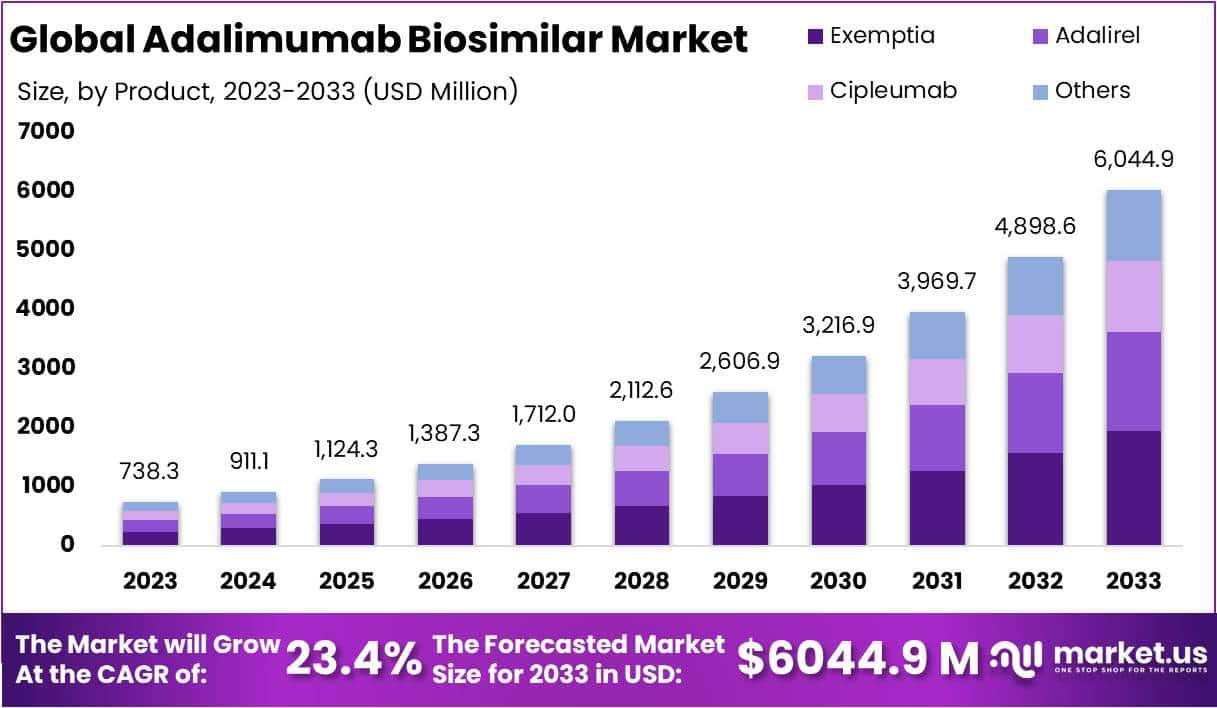
Key Takeaways
- The Adalimumab Biosimilar market is projected to reach USD 6,044.9 million by 2033, growing at a CAGR of 23.4% from 2024-2033.
- Exemptia led the market with a 32.1% share in 2023, driven by its advanced formulation, effectiveness, and competitive pricing.
- Hospital pharmacies accounted for 32.1% of the market share in 2023, providing easy access within integrated healthcare systems.
- Retail pharmacies showed consistent growth, becoming essential sources for Adalimumab Biosimilar medications, offering accessibility to patients.
- Key drivers include the rise in chronic diseases, patent expirations, cost-effective healthcare solutions, and supportive regulatory frameworks.
- The market faces challenges like complex manufacturing processes, stringent regulatory requirements, and limited patient awareness, which could hinder growth.
- Expansion into emerging markets, strategic partnerships, technological advancements, and broader therapeutic indications offer significant growth potential.
- Trends include biosimilar lifecycle management, patient assistance programs, distinctive branding strategies, and a shift toward self-administered biosimilars.
- In 2023, North America held the largest share (43.5%, USD 172.7 million), while Asia Pacific demonstrates the highest growth potential in the market.
Regional Analysis
In 2023, North America led the adalimumab biosimilars market with a dominant share of over 43.5%. The region achieved a market value of USD 172.7 million. This success is largely due to the well-established healthcare system and favorable reimbursement policies, particularly in the United States. These factors have driven the adoption of biosimilar drugs. The high prevalence of autoimmune diseases, such as rheumatoid arthritis and inflammatory bowel disease, further supports the demand for adalimumab as a primary treatment option.
The early launch of adalimumab biosimilars, such as Amjevita and Cyltezo, has also contributed to the strong market growth in North America. Additionally, the high costs associated with branded adalimumab have incentivized the shift toward biosimilars. This shift is supported by robust healthcare spending, a mature biopharmaceutical industry, and the presence of leading pharmaceutical players in the region. These factors create a favorable environment for the biosimilars market to expand further.
Europe is the second-largest market for adalimumab biosimilars, with an expected market value of approximately USD 112.6 million in 2023. The region benefits from supportive biosimilar regulations and patent expirations. Lower prices for biosimilars compared to branded biologics have encouraged adoption. Countries like Germany, the UK, and France are actively promoting biosimilar use, which has bolstered market growth. Strong government support and initiatives aimed at increasing biosimilar uptake have played a key role in the region’s success.
The Asia Pacific region is poised for rapid growth, with a projected compound annual growth rate (CAGR) of 27.4% from 2023 to 2030. The large patient pool, rising healthcare spending, and increasing disposable incomes make Asia Pacific a lucrative market for global companies. As more countries in the region implement regulations and guidelines for biosimilar approval, the growth prospects for adalimumab biosimilars remain strong. This expanding market offers significant opportunities for investment and market penetration.
Emerging Trends
- Market Entry and Competition: Since 2023, the U.S. FDA has approved several adalimumab biosimilars, such as Amjevita, Cyltezo, and Hyrimoz. These approvals bring more competition to the market. The goal is to lower treatment costs and expand access for patients. The increasing availability of these biosimilars marks a turning point in affordable healthcare options.
- Interchangeability Designations: Cyltezo (adalimumab-adbm) is the first biosimilar granted interchangeability status with Humira. This means pharmacists can substitute it without physician approval. This designation is likely to encourage more widespread adoption of biosimilars by simplifying the process for patients and healthcare providers.
- Pricing Strategies: Manufacturers have adopted aggressive pricing to capture the market. For instance, Boehringer Ingelheim offers Cyltezo through GoodRx at a 92% discount compared to Humira’s list price. These strategies are focused on affordability and accessibility, helping patients manage treatment costs.
- Insurance Coverage Shifts: Pharmacy benefit managers (PBMs) are driving change in the biosimilar market. Companies like Cigna’s Express Scripts and CVS Health’s Caremark plan to remove Humira from preferred drug lists. They will replace it with more cost-effective biosimilars starting in 2025. This shift is expected to significantly boost biosimilar usage in the coming years.
- Regulatory Developments: The FDA is proposing to remove the need for switching studies for biosimilars seeking interchangeability. This change would simplify the approval process and make it easier for manufacturers to bring more biosimilars to the market. As a result, the development and adoption of these drugs may accelerate.
Use Cases
- Rheumatoid Arthritis (RA): Adalimumab biosimilars are a cost-effective treatment option for patients with rheumatoid arthritis (RA). These biosimilars help reduce the financial burden on patients, potentially improving treatment adherence. In 2023, the net price for adalimumab prescriptions decreased by 43%, from $5,007 in Q4 2022 to $2,837 in Q4 2023. This reduction in price makes it easier for more patients to access this treatment, improving overall patient outcomes and quality of life.
- Psoriatic Arthritis and Plaque Psoriasis: Biosimilars for adalimumab are effective in treating psoriatic arthritis and plaque psoriasis. These conditions can cause painful symptoms and long-term skin damage. Adalimumab biosimilars help reduce inflammation and slow disease progression. The availability of more affordable options allows for greater patient access, leading to better management of the conditions and improved quality of life for many patients.
- Ankylosing Spondylitis: Patients with ankylosing spondylitis benefit from adalimumab biosimilars. These treatments provide relief from spinal inflammation and pain, which are hallmark symptoms of the disease. Biosimilars offer similar therapeutic effects as the original biologic drug, making them an effective alternative for managing the condition. The reduced cost of biosimilars also makes them a more accessible treatment option for patients.
- Inflammatory Bowel Diseases (IBD): Adalimumab biosimilars are used to treat inflammatory bowel diseases, including Crohn’s disease and ulcerative colitis. These conditions cause chronic inflammation in the digestive tract. Biosimilars help control the inflammation, leading to symptom relief and potential disease remission. The lower cost of biosimilars increases patient access to effective treatment, ultimately improving their quality of life.
- Juvenile Idiopathic Arthritis (JIA): For pediatric patients with juvenile idiopathic arthritis (JIA), adalimumab biosimilars offer a promising treatment option. These biosimilars are effective in managing the symptoms of JIA, which can include joint pain and inflammation. By providing a more affordable alternative, biosimilars reduce the economic burden on families while ensuring that children have access to necessary treatments for long-term management.
Conclusion
In conclusion, the adalimumab biosimilar market is poised for substantial growth, driven by factors such as patent expirations and increased demand for cost-effective treatments. Strategic collaborations and favorable regulatory changes are enhancing market accessibility and encouraging wider adoption of these alternatives. As biosimilars become more accepted, they offer significant cost savings and increased treatment options for patients with chronic diseases. Moving forward, the focus on developing markets, innovative pricing strategies, and expanded indications for use will likely fuel further expansion and competitiveness within this sector. This market evolution presents promising opportunities for stakeholders to leverage biosimilar benefits, improving patient outcomes while managing healthcare costs effectively.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



