Table of Contents
Overview
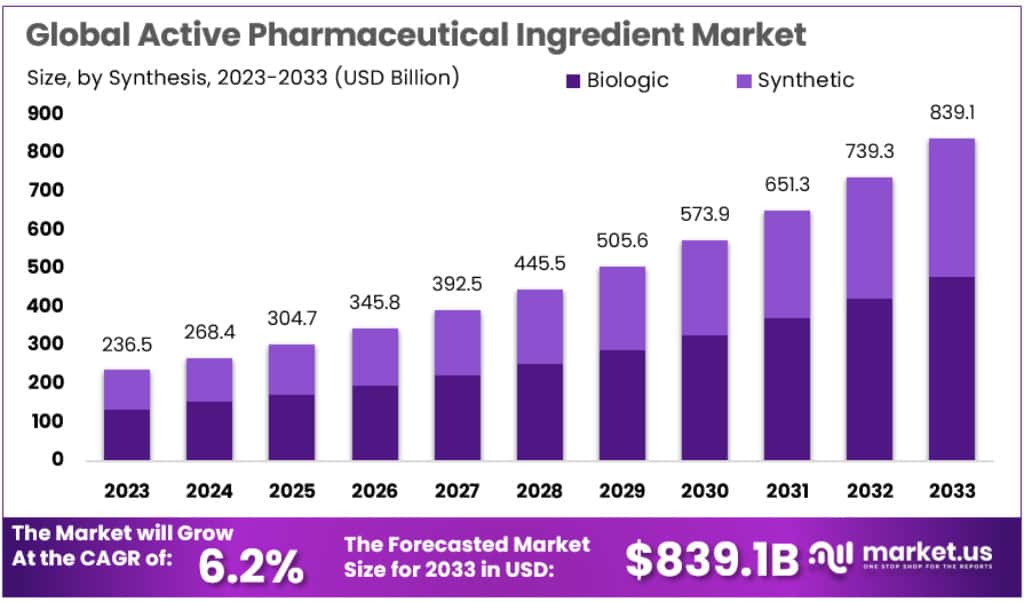
The Global Active Pharmaceutical Ingredient (API) Market is expected to grow significantly, reaching USD 839.1 billion by 2033 from USD 236.5 billion in 2023, at a CAGR of 6.2%. Several factors are driving this expansion, including increasing demand for generic drugs, technological advancements in manufacturing, and rising investments in biotechnology.
A major factor propelling the API market is the rising demand for generic drugs. The expiration of patents for many branded drugs has spurred the growth of generic alternatives, which are more affordable for consumers. This is particularly beneficial in markets where healthcare costs are a significant concern. Generic drugs are now widely used across both developed and emerging economies, increasing the demand for the APIs that form the core of these drugs.
Technological advancements have significantly transformed API manufacturing. Continuous manufacturing processes, which offer higher efficiency and scalability, are replacing traditional batch processes. This shift not only reduces production costs but also improves yields and minimizes waste. In addition, new synthesis techniques like biocatalysis and flow chemistry enable the production of complex APIs, making drug formulations more effective. These innovations contribute to the overall growth and competitiveness of the API market.
The API industry is heavily influenced by evolving regulatory frameworks. Agencies such as the U.S. FDA and the European Medicines Agency (EMA) continue to raise safety and quality standards for API production. Manufacturers are required to comply with these regulations, ensuring higher quality and safety in pharmaceutical products. Stricter standards push for the development of more robust production methods, driving both innovation and market growth.
Expanding Market Dynamics and Emerging Trends in the API Sector
The API sector is benefiting from several additional factors, such as increased outsourcing, rising healthcare demand in emerging markets, and the shift toward personalized medicine. These trends are expected to sustain growth and further shape the market’s trajectory in the coming years.
The trend of outsourcing API production to Contract Manufacturing Organizations (CMOs) has become increasingly prevalent. By outsourcing, pharmaceutical companies can reduce operational costs and focus on their core competencies, while CMOs handle complex API manufacturing. This shift toward outsourcing has enabled pharmaceutical companies to meet growing demand efficiently and at scale. As a result, CMOs play a crucial role in driving the API market’s growth.
Emerging economies, especially in the Asia Pacific region, are seeing rapid growth in their pharmaceutical sectors. As healthcare infrastructure expands and healthcare spending increases in these regions, the demand for APIs is also growing. Countries like India and China have become key global suppliers of APIs due to their cost-effective manufacturing capabilities. These regions have a well-established network of API producers, making them vital players in the global market.
Another important trend is the growing emphasis on personalized medicine. This approach tailors treatments to individual patient needs based on their genetic profile. To meet the requirements of personalized therapies, there is a rising demand for APIs that can cater to specific therapeutic needs. As precision medicine advances, pharmaceutical companies are increasingly developing specialized APIs, which are expected to become a major growth driver in the API market.
The growth of the API market is propelled by various factors, including the demand for generic drugs, technological innovations, regulatory advancements, and the expansion of healthcare in emerging markets. As the industry adapts to the rise of personalized medicine and outsourcing, the market is set to experience robust growth. The continuous evolution of the API sector reflects broader healthcare trends and is expected to thrive in the coming decade.
Key Takeaways
- The API market is projected to increase from USD 236.5 billion in 2023 to USD 839.1 billion by 2033, with a CAGR of 6.2%.
- Synthetic APIs hold a dominant share of 68.9% in the global market in 2023, outpacing biologic APIs.
- While biologic APIs have a smaller market share, they are experiencing strong growth due to their increasing demand in therapeutic applications.
- The cardiovascular diseases segment leads API applications, accounting for 22.1% of the market share in 2023, reflecting its critical healthcare importance.
- North America holds the largest API market revenue share at 39.5% in 2023, driven by high demand and advanced healthcare infrastructure.
- Asia Pacific is expected to grow at the fastest rate in the API market, with a projected CAGR of 8.4% over the next decade.
- Europe is also expected to see significant growth in the API market, fueled by increasing healthcare needs and pharmaceutical advancements.
- Innovative APIs represent over 54% of the market share in 2023, showcasing strong demand for new, advanced therapeutic solutions.
- Generic APIs continue to maintain a substantial market share, driven by their cost-effectiveness and widespread use for treating various conditions.
- The US, Canada, and Mexico are key contributors to the North American API market, due to established pharmaceutical sectors and demand for APIs.
- India and China are major players in the Asia Pacific, leveraging cost-efficient production capabilities to meet growing global demand for APIs.
Regional Analysis
In 2023, North America dominated the Active Pharmaceutical Ingredient (API) market, accounting for 39.5% of the revenue. This dominance is driven by the increasing prevalence of cancer and other lifestyle-related diseases, which fuels investment in pharmaceutical R&D. The market is expected to maintain this strong position, with a projected value of 93.4 billion USD. The ongoing demand for innovative treatments in the region is expected to support continuous market growth through the forecast period.
Asia Pacific is forecast to experience the highest growth, with a compound annual growth rate (CAGR) of 8.4%. Key economies like China and India contribute significantly to the market’s expansion by producing APIs at lower costs. Rising healthcare spending in these countries further supports the market’s growth. This cost efficiency and increased investment in healthcare infrastructure are driving the demand for active pharmaceutical ingredients in the region.
Europe is also expected to witness significant market growth during the forecast period. This growth is supported by increased research funding and the local presence of major pharmaceutical companies. The expansion of European companies in the pharmaceutical sector, along with rising investments, is expected to fuel the market. As these companies continue to grow, Europe will play an increasingly important role in the global API market.
Segmentation Analysis
In 2023, the Synthetic API segment dominated the market with a share of over 68.9%. This leadership is driven by the extensive portfolio of generics and advanced chemical synthesis technology. Synthetic APIs benefit from streamlined regulatory pathways and cost-effective manufacturing processes, which ensure their strong market presence. In contrast, the Biologic API segment, while smaller, is growing rapidly. Advances in biotechnology and the rise of biosimilars are fueling this growth, driven by patent expirations for blockbuster biologics and increasing demand for targeted treatments.
Cardiovascular diseases led the API market in 2023, holding a share of over 22.1%. The high global prevalence of cardiovascular conditions and the introduction of innovative therapies contributed to this dominance. Oncology APIs are also experiencing growth, fueled by increased cancer cases and heavy investment in research. The CNS and neurology segment, focused on disorders like Alzheimer’s and Parkinson’s, is gaining momentum as the aging population grows. Other segments like orthopedic, endocrinology, and pulmonology also show promising growth due to rising health concerns in these areas.
In 2023, Innovative APIs captured more than 54% of the market, benefiting from strong patent protections and high investment in novel treatments. The demand for these APIs is driven by the need for advanced therapeutics for complex diseases. On the other hand, Generic APIs hold a significant share, bolstered by patent expirations and a global push for cost-effective treatments. The increasing need for affordable medication in emerging markets is propelling the growth of generics. Both segments are expected to grow as innovation and affordability continue to shape the market.
Key Players Analysis
The market for highly potent active pharmaceutical ingredients (HPAPIs) is complex, driven by several factors. Patent expirations of blockbuster drugs and rising manufacturing costs are leading to increased outsourcing activities. The strict regulations governing the production of APIs further intensify competition within the market. These regulations ensure that only companies with significant resources and expertise can successfully operate, making the market challenging for new entrants. This competitive environment is expected to continue throughout the forecast period.
To retain their market position, major players in the HPAPI sector are focused on launching new products and expanding their portfolios. For instance, in 2021, Teva Pharmaceuticals and MEDinCell received U.S. FDA approval for a new medication aimed at treating schizophrenia. However, the approval process for new drugs remains complex, and legal challenges can delay progress. These hurdles slow down the establishment of new API production facilities and contribute to the overall market complexity.
Several prominent companies dominate the global HPAPI market, with Teva Pharmaceuticals, Dr. Reddy’s Laboratories, and Eli Lilly being key players. In 2020, these companies faced regulatory hurdles from the U.S. FDA regarding their API plants, highlighting the challenges of compliance. Given the substantial capital investment required, it is difficult for smaller companies to compete. As a result, the market remains largely concentrated among major players, limiting opportunities for new market entrants.
FAQ
1. What is an Active Pharmaceutical Ingredient (API)?
An Active Pharmaceutical Ingredient (API) is the substance in a drug that causes the intended therapeutic effect. It is responsible for treating, diagnosing, or preventing a disease. APIs are the core ingredients in pharmaceutical products, making them effective for medical use. Without the API, the drug would not provide the desired medical benefits. APIs are carefully tested and regulated to ensure they are safe and effective for consumers when used in appropriate doses.
2. What is the role of an API in a drug?
The role of an API is crucial as it provides the desired therapeutic effect in a medication. The API interacts with the body to produce specific actions, such as relieving pain, lowering blood pressure, or curing infections. It is the key ingredient that makes a drug effective. When a drug is consumed, the API works by targeting specific biological pathways. Excipients, which are other substances in the drug, help deliver the API to the intended site in the body.
3. How are APIs manufactured?
APIs are manufactured through two main methods: chemical synthesis and biotechnology. Chemical synthesis involves chemical reactions to create small molecule APIs, such as aspirin. Biotechnology uses living organisms or cells to produce biologic APIs, like monoclonal antibodies. Both methods follow strict guidelines to ensure quality and purity. The production of APIs must meet regulatory standards set by authorities like the FDA or EMA. Each method involves careful monitoring to maintain consistency and efficacy in the final product.
4. What is the difference between an API and an excipient?
An API is the active component of a drug responsible for its therapeutic effect. In contrast, excipients are inactive substances used in drug formulations. Excipients help with the drug’s stability, delivery, and absorption but do not provide any therapeutic benefit. Examples of excipients include fillers, binders, and preservatives. While the API delivers the medical benefit, excipients ensure the drug is safe, effective, and easy to administer. Together, both components form the complete pharmaceutical product.
5. What are the different types of APIs?
There are two main types of APIs: small molecule APIs and biologic APIs. Small molecule APIs are chemically synthesized and include substances like ibuprofen or aspirin. These are typically used in oral tablets, capsules, or injections. Biologic APIs, on the other hand, are derived from living organisms and are used in biologic drugs. These include monoclonal antibodies, vaccines, and gene therapies. Both types of APIs are crucial in modern medicine and are developed using advanced technologies for different therapeutic purposes.
6. What are the regulatory requirements for APIs?
APIs must meet strict regulatory requirements set by agencies like the FDA, EMA, and WHO. Manufacturers are required to submit detailed documentation about the API’s quality, safety, and manufacturing process. This includes information on sourcing raw materials, production processes, and quality control tests. Regulatory authorities review this data to ensure that APIs are safe for human use. Compliance with these regulations is essential for the approval of new drugs and to ensure consistency and quality in mass production.
7. Why are APIs important in drug development?
APIs are the cornerstone of drug development. They are responsible for delivering the therapeutic effects that treat diseases or symptoms. The development of a new API involves identifying a substance with the potential to address a specific medical need. Once identified, the API is tested for safety, efficacy, and stability. Without an effective API, a drug would have no medical benefit. Therefore, the API plays a critical role in the development and success of pharmaceutical products.
8. What are the challenges in the API industry?
The API industry faces several challenges, including complex regulatory requirements, high production costs, and supply chain issues. Ensuring consistent quality is a major concern, especially for biologics and complex molecules. Additionally, the increasing demand for generic drugs puts pressure on manufacturers to keep costs low while maintaining quality. The industry must also adapt to evolving regulations and ensure compliance with global standards. These challenges require constant innovation and strategic planning to remain competitive and meet market demands.
9. What is the Active Pharmaceutical Ingredient (API) market?
The API market refers to the global industry that produces the active ingredients in pharmaceutical products. This market includes the production of both small molecule and biologic APIs. It plays a vital role in the pharmaceutical sector as APIs are the core components of all medications. The API market encompasses manufacturing, distribution, and regulatory compliance. Growing healthcare needs, rising chronic diseases, and the demand for generics are key factors driving the expansion of the API market.
10. What is driving the growth of the API market?
Several factors are driving the growth of the API market. Rising healthcare demands due to aging populations and chronic diseases are key drivers. The shift toward generic drugs, as patents on branded drugs expire, has also contributed to market expansion. Moreover, advancements in biotechnology have led to the development of biologic drugs, fueling the growth of biologic APIs. Increasing healthcare expenditure in emerging markets is another factor. These trends are expected to continue shaping the future of the API market.
11. Which region dominates the API market?
Asia-Pacific (APAC) currently dominates the API market, with countries like India and China being major manufacturing hubs. The region offers cost-effective production and has a strong export market. India, in particular, is known for producing high-quality generic APIs at competitive prices, making it a key player in the global market. Other regions, such as North America and Europe, also contribute to the market but at a smaller scale compared to APAC, which continues to lead in both production and supply.
12. What are the types of APIs in the market?
The API market is primarily divided into two types: generic APIs and innovative APIs. Generic APIs are used in drugs that are produced once the patent for the original branded drug expires. These are typically less expensive and more accessible. Innovative APIs, on the other hand, are used in new drugs that are still under patent. These APIs are typically more complex and are used in advanced therapies. Both types are essential to the overall pharmaceutical market, with generics making medications more affordable.
13. What are the major players in the API market?
The API market is dominated by key players such as Merck & Co. Inc., AbbVie Inc., Bristol-Myers Squibb Company, Boehringer Ingelheim International GmbH, Cipla Inc., Teva Pharmaceutical Industries Ltd., Albemarle Corporation, Viatris Inc., Aurobindo Pharma, Sun Pharmaceutical Industries Ltd., Dr. Reddy’s Laboratories Ltd., and Other Key Players. These companies are involved in manufacturing, developing, and supplying APIs to pharmaceutical companies worldwide. They lead the market in both the production of small molecule APIs and biologic APIs. Many of these companies also focus on expanding their manufacturing capabilities, especially in emerging markets, to meet the growing demand for high-quality, cost-effective APIs.
14. How is the COVID-19 pandemic affecting the API market?
The COVID-19 pandemic has significantly impacted the API market, causing disruptions in supply chains and production. Lockdowns and travel restrictions led to delays in the sourcing of raw materials and finished APIs. At the same time, there was an increased demand for certain APIs used in COVID-19 treatments and vaccines. These disruptions highlighted the need for stronger, more resilient supply chains. Moving forward, the pandemic has encouraged companies to diversify production and reduce dependency on a few regions for critical API supplies.
15. What are the trends in the API market?
Several key trends are shaping the API market. There is a growing focus on biologics, driven by advancements in biotechnology and the rise of targeted therapies. Another trend is the increasing demand for generic APIs as more branded drugs lose patent protection. Outsourcing API production to contract manufacturing organizations (CMOs) is also becoming more common, as it helps pharmaceutical companies reduce costs and focus on core activities. Additionally, there is a greater emphasis on regulatory compliance and sustainability in API manufacturing processes.
16. What is the future outlook for the API market?
The future of the API market looks promising, driven by the increasing global demand for both generic and biologic drugs. With advancements in biotechnology, new APIs are being developed to address unmet medical needs. The aging population and the rise of chronic diseases will continue to fuel the demand for pharmaceuticals. Moreover, emerging markets, particularly in Asia-Pacific, will see growing demand for high-quality, affordable APIs. The market is expected to expand steadily, with increased focus on sustainability and regulatory compliance in production.
17. How are environmental regulations impacting the API market?
Environmental regulations are becoming more important in the API market. Manufacturers are required to comply with standards related to waste management, emissions, and energy use. Stricter environmental guidelines are pushing companies to adopt more sustainable manufacturing processes. These regulations ensure that API production has minimal environmental impact. Many companies are investing in greener technologies and practices to meet these standards and avoid penalties. Compliance with environmental regulations is essential for long-term sustainability in the API industry.
18. What are the challenges faced by API manufacturers?
API manufacturers face several challenges, including stringent regulatory requirements, high production costs, and supply chain disruptions. Meeting global quality standards while maintaining cost efficiency is a constant balancing act. Manufacturers must also cope with raw material shortages and navigate the complexities of biologic drug production. Furthermore, increasing competition in the market puts pressure on pricing. To stay competitive, manufacturers must focus on innovation, regulatory compliance, and building resilient supply chains that can withstand external disruptions.
Conclusion
In conclusion, the Active Pharmaceutical Ingredient (API) market is poised for substantial growth due to the rising demand for generic drugs, technological advancements, and increased investments in biotechnology. Key factors such as regulatory improvements, the shift towards personalized medicine, and outsourcing of API production are driving this expansion. The market will continue to evolve, with significant growth expected in emerging regions like Asia-Pacific. With innovative therapies gaining traction and the need for cost-effective solutions increasing, the API market is set to thrive in the coming years, offering opportunities for both established and new players in the industry.
View More
Biopharmaceuticals Market || Radiopharmaceuticals Market || Pharmaceutical Excipients Market || Pharmaceutical Cartridges Market || Generic Pharmaceuticals Market || NanoPharmaceuticals Market || Pharmaceutical Filtration Market || Active Pharmaceutical Ingredient Market || Specialty Pharmaceutical market || Pharmaceutical Grade Lactose Market || Asia-Pacific Active Pharmaceutical Ingredient Market || High Potency Active Pharmaceutical Ingredients Market || Pharmaceutical Analytical Testing Market || Pharmaceutical Drug Delivery Market || Pharmaceutical Grade Washer Market || Pharmaceutical Manufacturing Software Market || Pharmaceutical Water Market || Generative AI In Pharmaceutical Market || Pharmaceutical Third-Party Logistics Market || Pharmaceuticals Market
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



