Table of Contents
Introduction
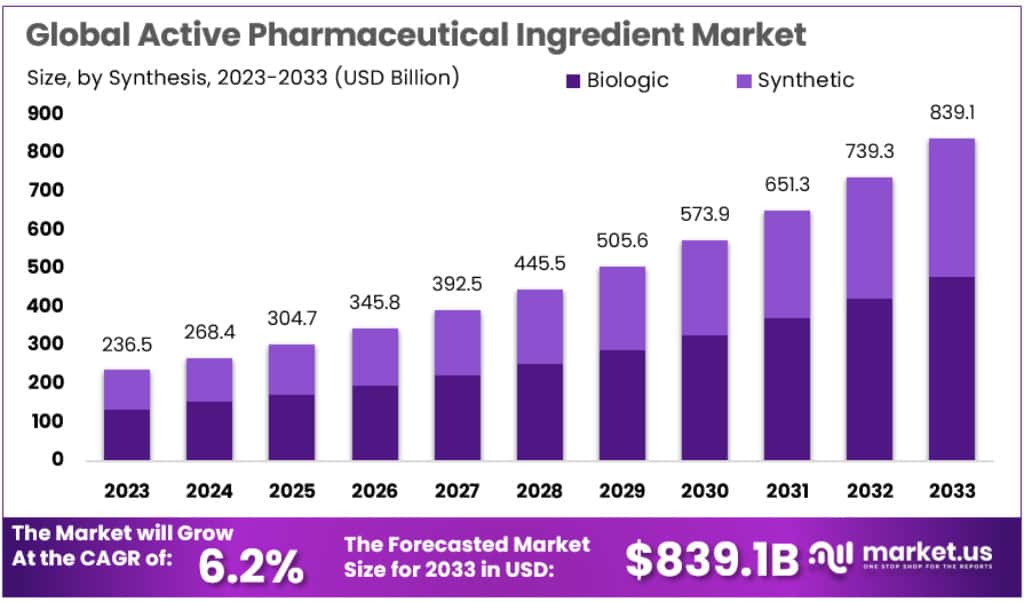
The Global Active Pharmaceutical Ingredient (API) market is projected to expand significantly, growing from USD 236.5 billion in 2023 to an estimated USD 839.1 billion by 2033, at a compound annual growth rate of 6.2%. This growth is fueled by a rising global demand for medications, driven by aging populations and an increase in chronic diseases, particularly in developing economies.
Technological innovations in biotechnology are transforming API development, with advancements in biopharmaceuticals, gene therapy, and specialized drugs enhancing treatment efficacy and broadening therapeutic possibilities. Additionally, streamlined regulatory processes are facilitating quicker approvals and launches of new therapies, especially for critical health conditions.
The API manufacturing sector is increasingly globalizing, with significant production shifts to countries like India and China due to lower production costs and improved manufacturing quality. The trend towards continuous manufacturing is enhancing operational efficiencies, reducing waste, and lowering costs, meeting stringent quality and regulatory standards more effectively.
Strategic outsourcing to Contract Manufacturing Organizations (CMOs) is another key growth driver, allowing pharmaceutical firms to concentrate on drug discovery and marketing while managing production complexities cost-effectively. This collaboration is pivotal in sustaining growth and innovation in the API sector, addressing both immediate and long-term healthcare needs globally.
Key Takeaways
- The API market is projected to expand from USD 236.5 billion in 2023 to approximately USD 839.1 billion by 2033.
- With a CAGR of 6.2%, the API market is set to experience steady growth from 2023 to 2033.
- Synthetic APIs dominate the market, holding a 68.9% share in 2023, leading the industry.
- Biologic APIs are gaining traction, showing robust growth despite a smaller market share compared to synthetic APIs.
- The cardiovascular diseases segment holds the largest share of API applications, accounting for 22.1% in 2023.
- North America holds the largest market revenue share, contributing 39.5% in 2023, driven by demand from the US, Canada, and Mexico.
- Asia Pacific is expected to witness the fastest growth, with a projected CAGR of 8.4%, led by key players in India and China.
- Europe is also projected to experience notable growth, contributing significantly to the expansion of the global API market.
- Innovative APIs make up over 54% of the market share in 2023, reflecting ongoing advancements in API development.
- Generic APIs continue to maintain a strong presence in the market, driven by the need for cost-effective treatment solutions.
Emerging Trends
- Sustainable Manufacturing Practices: The pharmaceutical industry is shifting towards eco-friendly methods for API production. Sustainable manufacturing is crucial to reducing environmental impact. Organizations like the World Health Organization (WHO) emphasize the importance of green practices in API research and development. These practices aim to lower energy consumption, reduce waste, and improve efficiency. By adopting these strategies, companies can minimize their carbon footprint while meeting regulatory requirements. The focus on sustainability is growing as governments and regulatory bodies promote cleaner and greener manufacturing solutions.
- Advanced Manufacturing Technologies: Innovative technologies like continuous manufacturing and process automation are transforming API production. These advancements improve efficiency, reduce costs, and enhance product quality. The U.S. Food and Drug Administration (FDA) actively supports the adoption of emerging technologies in pharmaceutical manufacturing. These technologies enable manufacturers to produce APIs with greater precision and consistency. As a result, pharmaceutical companies can meet the growing demand for high-quality APIs more effectively. Advanced manufacturing is also helping reduce production timelines and create a more reliable supply chain.
- Regulatory Focus on Quality Management: Global regulatory bodies are tightening guidelines to ensure consistent API quality. The FDA, for instance, has released updated guidance on good manufacturing practices (GMP). These regulations apply to APIs used in both human and veterinary medicines. By implementing stringent quality management systems, manufacturers can comply with international standards. This focus on quality ensures patient safety and reduces the risk of drug recalls. The trend highlights the growing importance of regulatory oversight in maintaining the integrity of the pharmaceutical supply chain.
- Increased Access to Complex Generics: Access to complex generics, which often involve intricate APIs, is improving. The FDA is working to validate new methods for comparing complex generics with reference drugs. These efforts aim to streamline the approval process while ensuring safety and efficacy. Complex generics are essential for reducing healthcare costs and providing more treatment options. With regulatory support, manufacturers are overcoming challenges in developing and producing these advanced formulations. The increased availability of complex generics benefits patients worldwide by improving access to affordable medications.
Use Cases
- Treatment of Chronic Diseases: Active Pharmaceutical Ingredients (APIs) are critical for managing chronic illnesses like diabetes and hypertension. For example, metformin is an essential API widely used in diabetes treatment. Its global production reaches thousands of metric tons each year to meet increasing patient demands. The effectiveness of APIs like metformin highlights their role in improving quality of life for people with chronic conditions. Ensuring consistent API quality is essential for maintaining treatment efficacy and safety for patients worldwide.
- Antibiotic Therapies: APIs like amoxicillin are indispensable in treating bacterial infections through antibiotics. They ensure effective outcomes when formulated according to stringent standards. Organizations like the World Health Organization (WHO) issue detailed guidelines for quality control in antibiotic APIs. These standards protect global health by ensuring antibiotics work effectively against infections. By maintaining high-quality APIs, the healthcare industry combats antimicrobial resistance and ensures safer treatments for millions.
- Oncology Treatments: Cancer medications heavily rely on specialized APIs to target specific types of cancer effectively. These APIs undergo rigorous development and quality assurance processes to ensure safety and efficacy. Regulatory bodies like the FDA provide strict guidelines for these APIs, focusing on patient safety and drug reliability. With advanced APIs, oncology treatments are more precise and effective, offering hope to patients. High-quality APIs are the backbone of innovative cancer therapies.
- Vaccines and Biologics: APIs are integral to producing vaccines and biologic therapies that prevent or treat serious illnesses. The FDA emphasizes the quality of APIs, even in alternative sourcing for unapproved drug applications. This ensures vaccines remain effective and safe for public use. APIs in biologics also require thorough quality control to meet stringent standards. Their importance cannot be overstated, as they help protect global health and combat emerging diseases.
Conclusion
The Active Pharmaceutical Ingredient (API) market is experiencing significant growth, driven by rising global demand for medications and advancements in technology. Biopharmaceutical innovations, streamlined regulations, and the increasing adoption of sustainable manufacturing practices are shaping the future of API production. Countries like India and China are emerging as key manufacturing hubs, enhancing global supply chains. The focus on advanced manufacturing technologies and quality management ensures improved efficiency and safer treatments. With growing applications in chronic disease management, cancer therapies, and vaccines, APIs are critical to modern healthcare. As the market evolves, collaboration and innovation will remain vital to addressing healthcare needs and delivering accessible, high-quality treatments globally.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



