mRNA Statistics: In recent years, mRNA technology has gained significant attention due to its potential applications in medicine. The development of mRNA-based vaccines such as the COVID-19 mRNA vaccines has demonstrated the effectiveness and versatility of this technology.
mRNA vaccines work by delivering synthetic mRNA molecules into cells, which then instruct the cells to produce harmless fragments of a pathogen. This, in turn, triggers an immune response leading to the production of protective antibodies against the pathogen.
The study of mRNA and its functions continues to advance our understanding of cellular processes and holds promise for future therapeutic and medical applications.
Table of Contents
- What is mRNA?
- mRNA Statistics – History of mRNA Vaccines
- mRNA Statistics – Pfizer- BioNTech Vaccine, BNT162b2
- mRNA Statistics – Moderna vaccine, mRNA-1273 Vaccine
- COVID-19 Vaccine Comparison Biontech/Pfizer vs Moderna 2020
- mRNA Statistics – Leading Pharmaceutical Products by Sales Worldwide 2022
- mRNA Statistics – Vaccines By Country
- mRNA Statistics – Leading Biotech Companies (March 2023)
- COVID-19 Vaccines and Drugs Worldwide in 1st HY 2021
- Recent Developments
- Wrap Up
- FAQs
What is mRNA?
mRNA (messenger RNA) is a type of RNA molecule that plays an important role in protein synthesis. mRNA carries genetic information from DNA in the nucleus of the cell to the ribosomes, which are the cellular structures responsible for protein production.
The process of protein synthesis involves two main steps transcription and translation. During transcription an enzyme called RNS polymerase helps create a complimentary copy of a specific DNA sequence, forming a pre-mRNA molecule.
This pre-mRNA undergoes further processing including the removal of non-coding regions called introns and the splicing together of coding regions called exons, resulting in a mature mRNA molecule.
Once the mRNA molecule is formed, it exits the nucleus and enters the cytoplasm of the cell. Where it serves as a template for protein synthesis.
The ribosomes read the sequence of nucleotides in the mRNA molecule and use it as instructions to assemble the corresponding amino acids in the correct order, forming a protein.
mRNA Statistics – History of mRNA Vaccines
The concept of mRNA vaccine was first developed in the 1990s, but due to a lack of technological advancements, delivery challenges, and inherent stability of mRNA.
The field of mRNA vaccine has been triggered from the past few decades of the COVID-19 pandemic. mRNA vaccines are used in the treatment of infectious diseases such as rabies, influenza, Zika, and HIV-1.
Soon after the emergence of coronavirus, Pfizer and BioNTch started developing mRNA vaccines, as did Moderna- the letter in partnership with the National Institute of Allergy and Infectious Diseases.

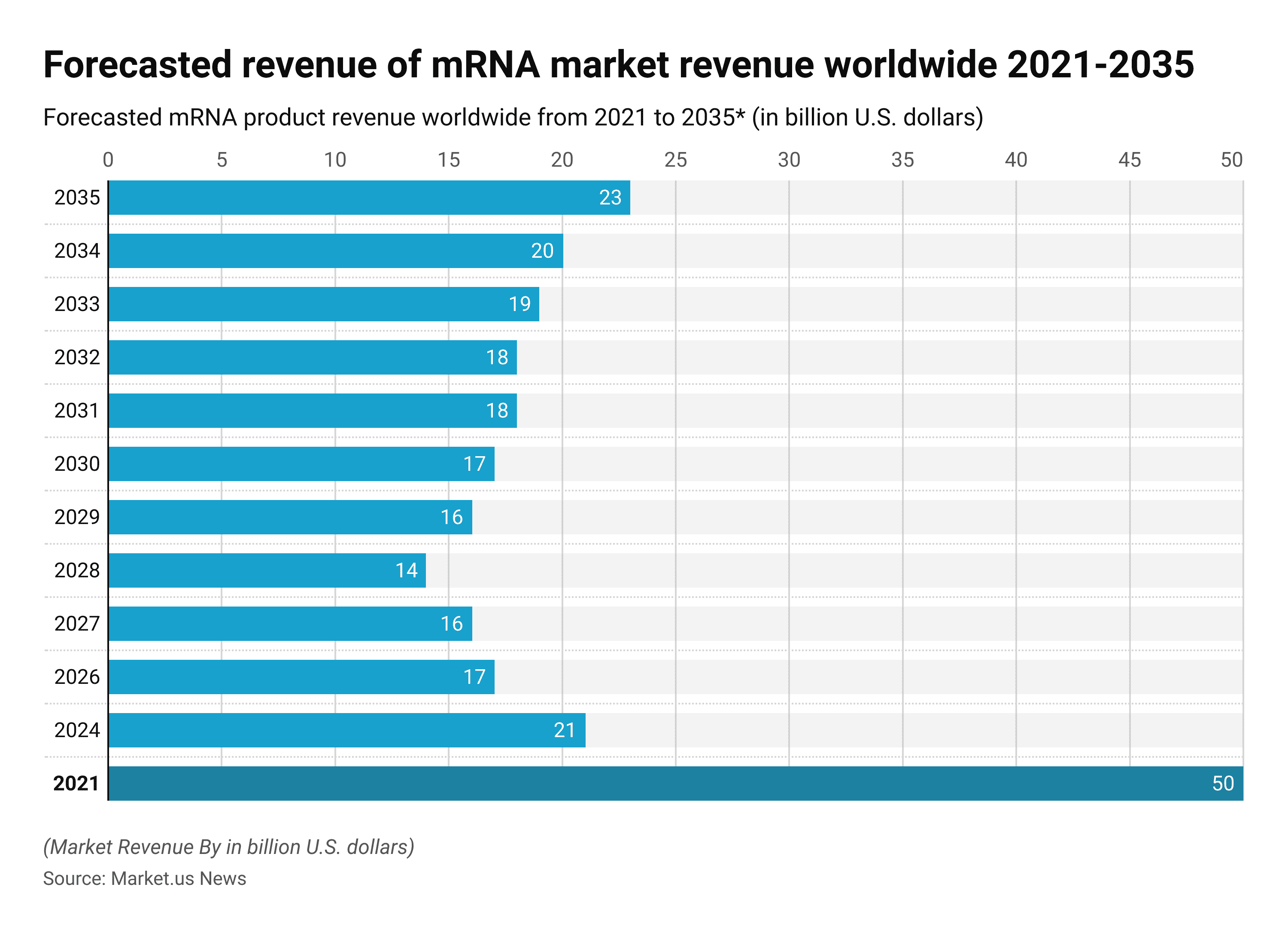
mRNA Statistics – Market Revenue Forecast Worldwide 2021-2035
The mRNA products have come into the market only due to COVID-19.
- In 2021, the revenue of the mRNA market was USD 50 billion and it is expected that the revenue will decrease to USD 23 billion by 2035.
- In 2022, the global RNA analysis market size was valued at USD 9.58 billion and is expected to reach USD 34.37 billion in 2032. This market is estimated to register a CAGR of 14% between 2023 and 2032.
This statistic illustrates the forecasted revenue of mRNA products worldwide from 2021 to 2035.
mRNA Statistics – Pfizer- BioNTech Vaccine, BNT162b2
Pfizer- BioNTech vaccine, BNT162b2 uses mRNA to create the receptor binding domain of the spike protein of SARS-CoV-2. Clinical trials involving tens of thousands of participants have shown that the Pfizer-BioNTech vaccine is highly effective in preventing symptomatic COVID-19. It has demonstrated efficacy rates of around 95% in preventing COVID-19 infection in individuals without prior exposure to the virus.
Efficacy
- The efficiency of Pfizer- BioNTech vaccine, BNT162b2 vaccine remains consistently above 70% in both the age group 6 to 23 months and ages 6 months to 4 years.
- The vaccine was 95% effective in protecting trial participants from COVID-19 for those 16 years and older.
- The vaccine was 100% effective for those 12 to 15 years old.
- 90.7% effective for those 5 to 11 years old.
(Source: Canada)
Common Side Effects
There have been no serious adverse events observed in the phase 3 clinical trial for Pfizer- BioNTech vaccine, BNT162b2. The mild local side effects are observed such as heat, pain, redness, and swelling.
The other systemic side effects including fatigue, fever, headache, myalgia, and arthralgias occur more frequently with the vaccine than with placebo, with most occurring within 1 to 2 days following vaccination.
- The most common reaction with the vaccine was pain at the injection site within one week of vaccination.
- The majority of common reactions are seen in individuals aged 26 years and above.
- Less than 1% of participants reported severe pain and it was most common in individuals aged 55 years and above.
- In the trials, the younger individuals aged 16 to 55 years reported systemic events.
- Most of the systemic reactions are seen in the second dose of COVID-19 as compared to the first dose.
- The incidence of systemic reactions was less than 1% in the first vaccine and less than 2% in the second vaccine.
- About 3.8% of individuals reported fatigue.
- 2% of individuals reported headaches.
- Only 0.2% of vaccine receivers and 0.1% of placebo recipients reported fever up to 40 °C in the first dose of vaccine.
- About 0.8% of vaccine recipients and 0.1% of placebo recipients reported fever up to 40 °C in the second dose of vaccine.
- Adverse events were more commonly reported among vaccine recipients (27 %) compared to the placebo group (12 %).
(Source: pssjournal)
mRNA Statistics – Moderna vaccine, mRNA-1273 Vaccine
Moderna vaccine, mRNA-1273 uses mRNA to create the SARS-CoV-2 spike protein stabilized in its prefusion conformation.
Clinical trials involving tens of thousands of participants have shown that the Moderna vaccine is highly effective in preventing symptomatic COVID-19. It has demonstrated efficacy rates of around 94-95% in preventing COVID-19 infection in individuals without prior exposure to the virus.
The COVID-19 vaccine mRNA-1273 later brands named Spikevax-was the company’s first product approved by the Federal Drug Administration.
Its massive use is seen in Western countries. Spikevax was among the world’s second bestselling pharmaceutical products in 2022, generating over USD 21 billion in revenues.
Efficacy
- The efficacy of the vaccine against COVID-19 was 36.8% among 2 to 5-year-olds.
- The efficacy of the vaccine among adults aged 6 to 23 months was 50.6% at the time when the omicron variant was the predominant circulating variant.
(Source: JAMA Network)
Common Side Effects
- Similar to the Pfizer-BioNTech vaccine, the Moderna vaccine can have side effects, although the majority of them are mild and temporary.
- Common side effects reported with the Moderna vaccine include pain or swelling at the injection site, fatigue, headache, muscle pain, chills, fever, and nausea. These side effects typically resolve within a few days.
Serious Adverse Events
- Serious adverse events associated with the Moderna vaccine are rare but they can occur. In rare instances, severe allergic reactions and anaphylaxis have been reported. However, the overall occurrence of anaphylaxis after receiving the Moderna vaccine is extremely low.
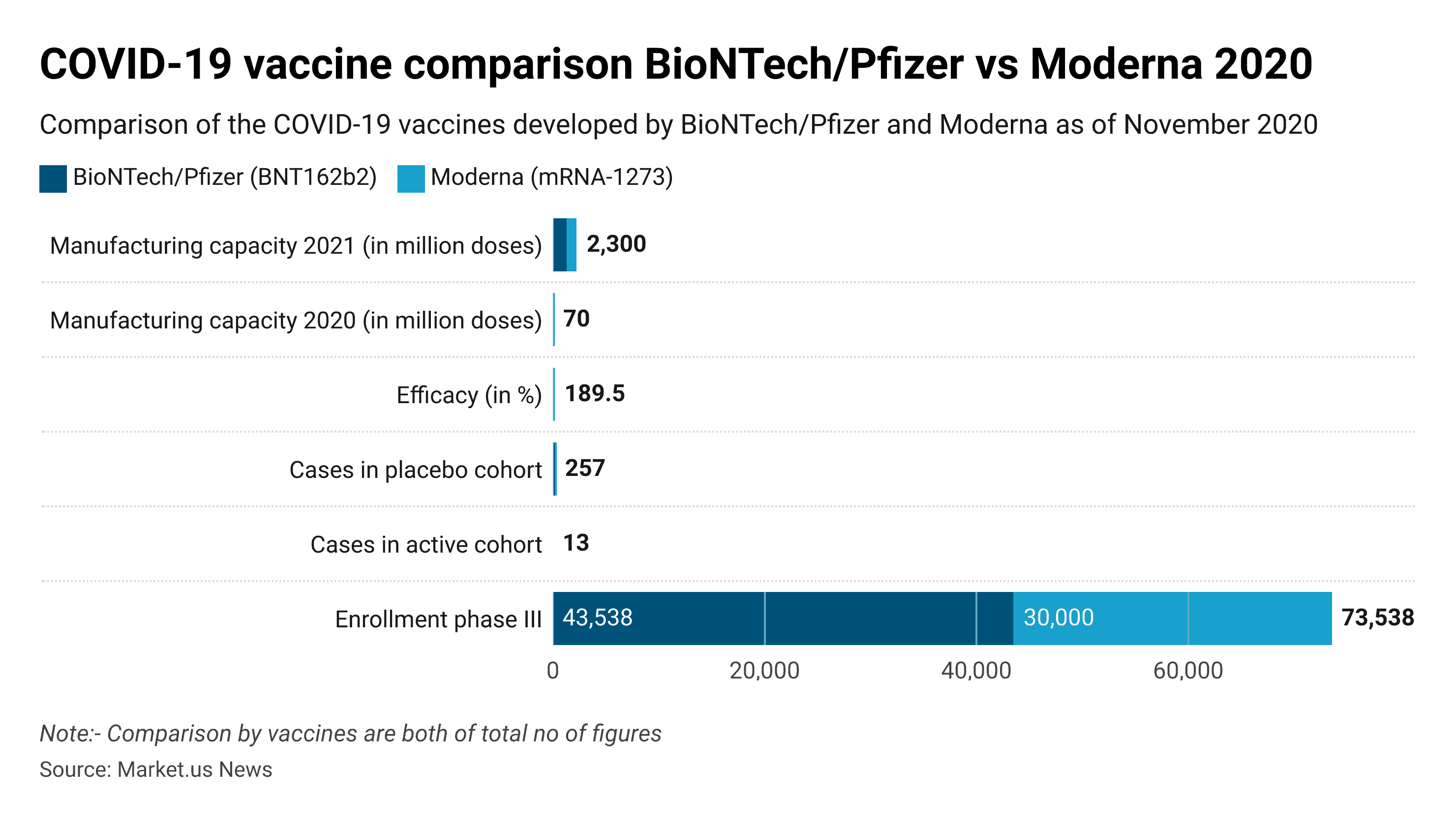
COVID-19 Vaccine Comparison Biontech/Pfizer vs Moderna 2020
- In November 2020, COVID-19 vaccines from the Biotech/Pfizer cooperation and Moderna showed very good results in phase III of clinical trials.
- Both sites reported nearly the same efficacy of around 95%.
- Pfizer and BioNTech will probably be able to produce larger amounts until year-end 2020 and also during 2021.
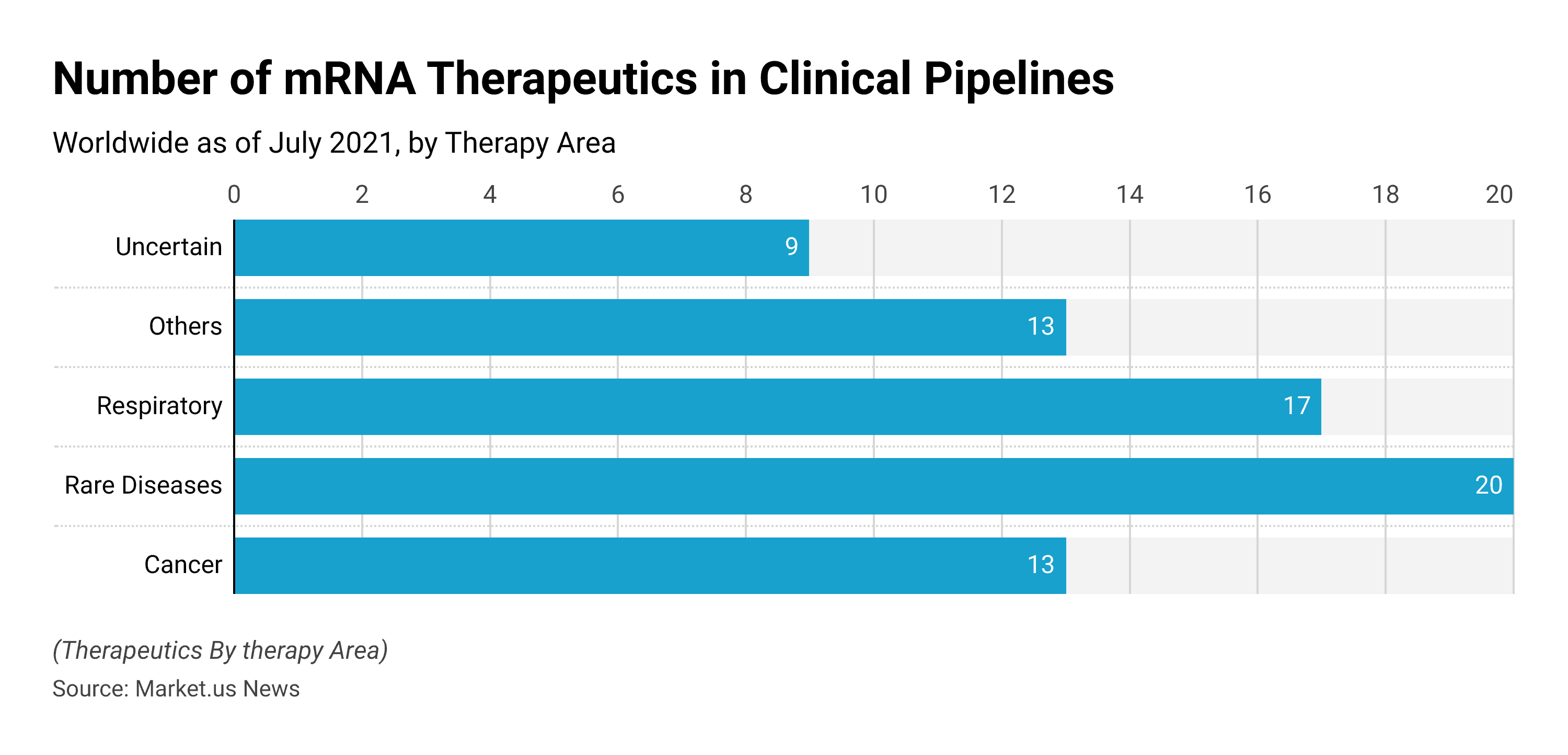
Number of mRNA therapeutics in clinical pipelines worldwide as of July 2021, by therapy area
- In 2021, there were around 20 mRNA therapeutics for rare diseases being developed in the clinical pipeline worldwide.
- There were around 17 mRNA therapeutics for respiratory diseases being developed in clinical pine lines worldwide.
- There were around 13 therapeutics for cancer being developed in the clinical pipeline worldwide.
This statistic illustrates the number of mRNA therapeutics in clinical pipelines worldwide as of July 2021, by therapy area.
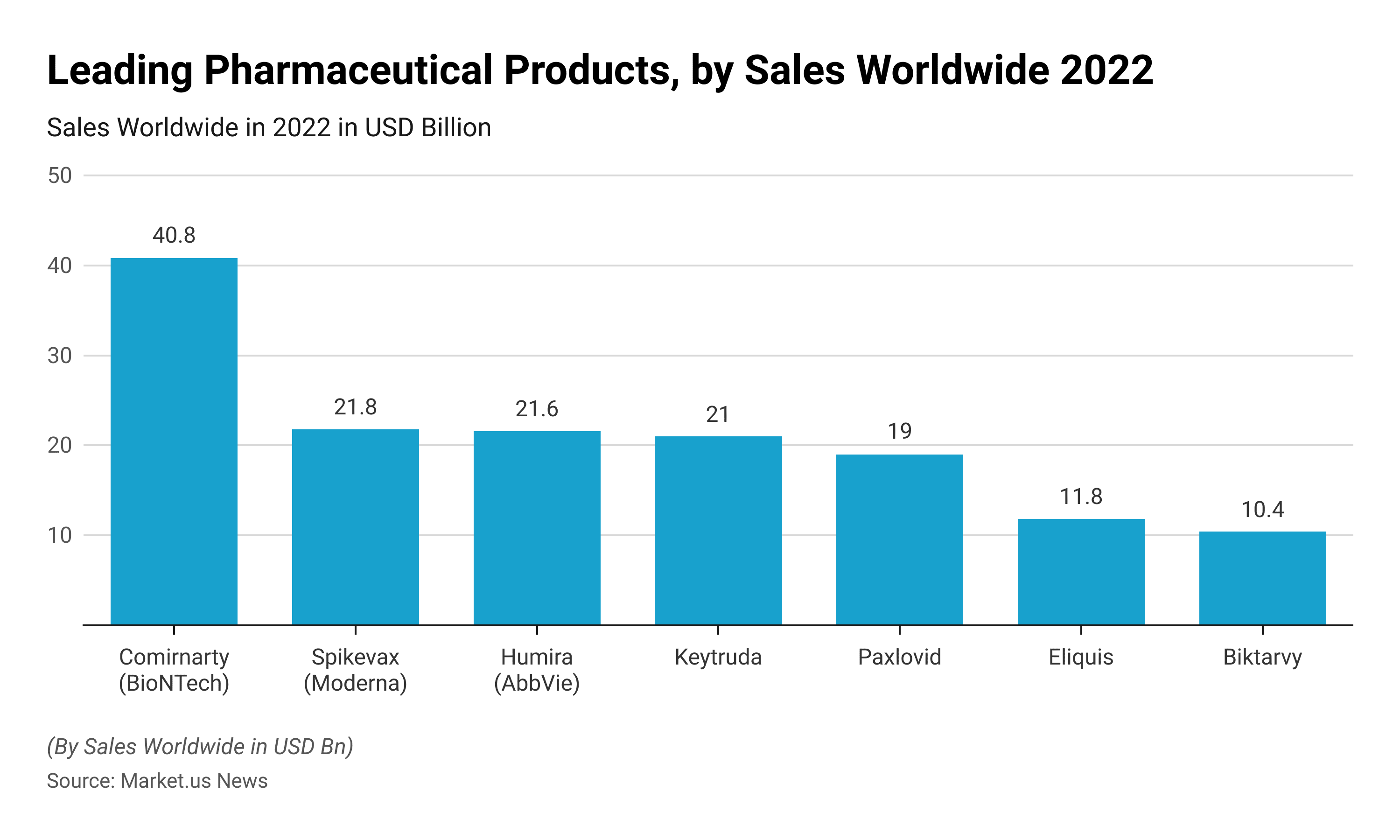
mRNA Statistics – Leading Pharmaceutical Products by Sales Worldwide 2022
- During the COVID-19 pandemic, the COVID-19 vaccine Comirnaty was top top-performing pharmaceutical product, generating a revenue of USD 40.8 billion.
- The Spikevax which is the Moderna COVID-19 vaccine was the second largest selling pharmaceutical product which generated revenue of USD 21.8 billion in 2022.
- Humira, Keytruda, and Paxlovid are the other most-selling pharmaceutical products worldwide in 2022.
mRNA Statistics – Vaccines By Country
United States
- The Pfizer-BioNTech vaccine received emergency use authorization (EUA) from the U.S. Food and Drug Administration (FDA) on December 11, 2020, for individuals aged 16 and older.
- Later, it was expanded to include individuals aged 12 to 15. The Moderna vaccine also received EUA from the FDA on December 18, 2020, for individuals aged 18 and older. Both vaccines require two doses administered several weeks apart.
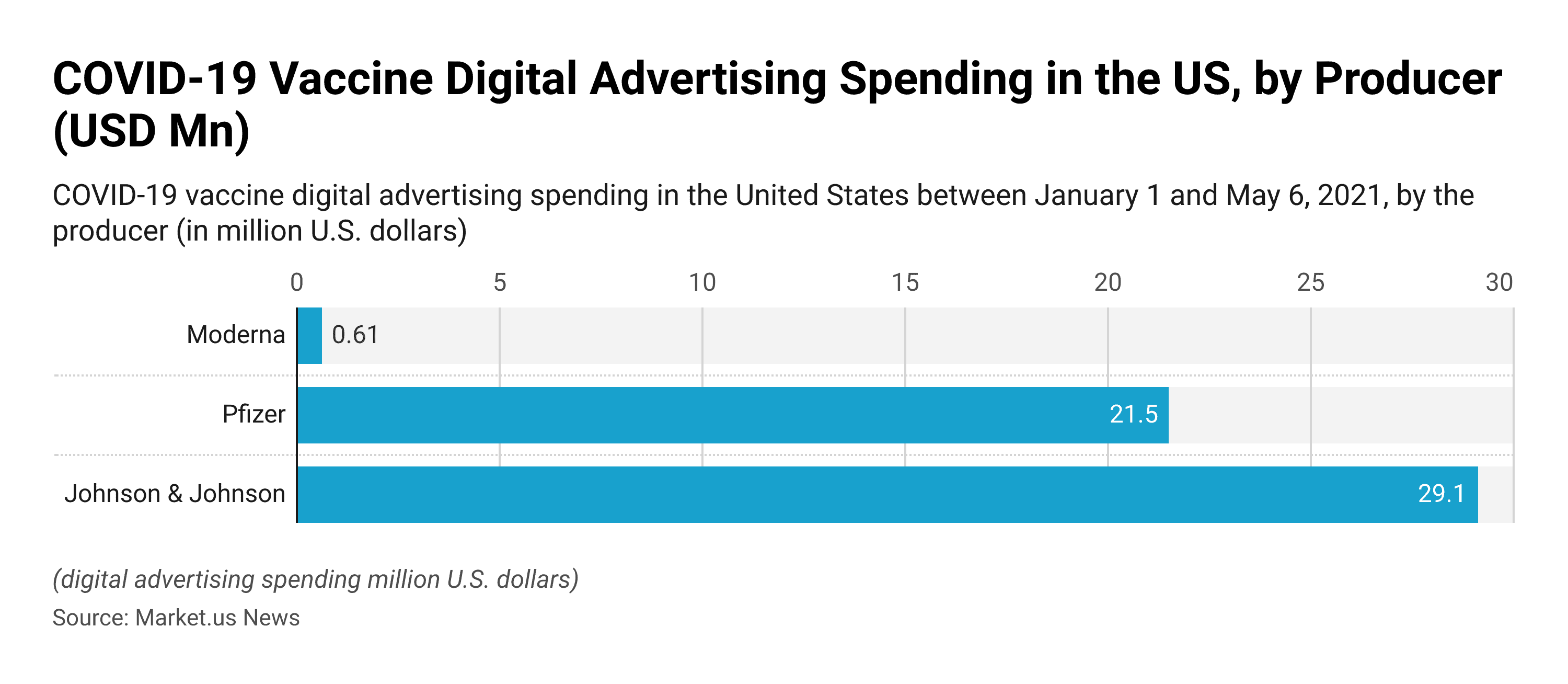
COVID-19 vaccine digital ad spend in the U.S. 2021, by producer
- Johnson & Johnson was the pharmaceutical company that spent the most in COVID-19 digital advertising spending in the United States between January 1 and May 6, 2021. The company’s spending amounted to 29.1 million U.S. dollars.
- Pfizer followed with 21.5 million.
- The launch of Pfizer’s mRNA vaccine in early December 2020 helped the pharma giant’s revenue during the first quarter of 2021 surge 45% from its revenue during the first quarter of 2020.
Canada
- In Canada, the Pfizer-BioNTech vaccine was the first mRNA vaccine authorized for emergency use. It received regulatory approval from Health Canada on December 9, 2020, for individuals aged 16 and older.
- Subsequently, Health Canada granted authorization for individuals aged 12 to 15 to receive the Pfizer-BioNTech vaccine.
- The Moderna vaccine also received regulatory approval from Health Canada on December 23, 2020, for individuals aged 18 and older.
Share of the Canadian population by COVID vaccination status, March 26, 2023
- About 83.4% of the Canadian population had received at least one COVID-19 vaccination dose.
- 80.7% of the population in Canada received primary series COVID-19 vaccines as of March 26, 2023.
- Only 19% of the population in Canada received booster doses in the last 6 months.
(Source: Statista)
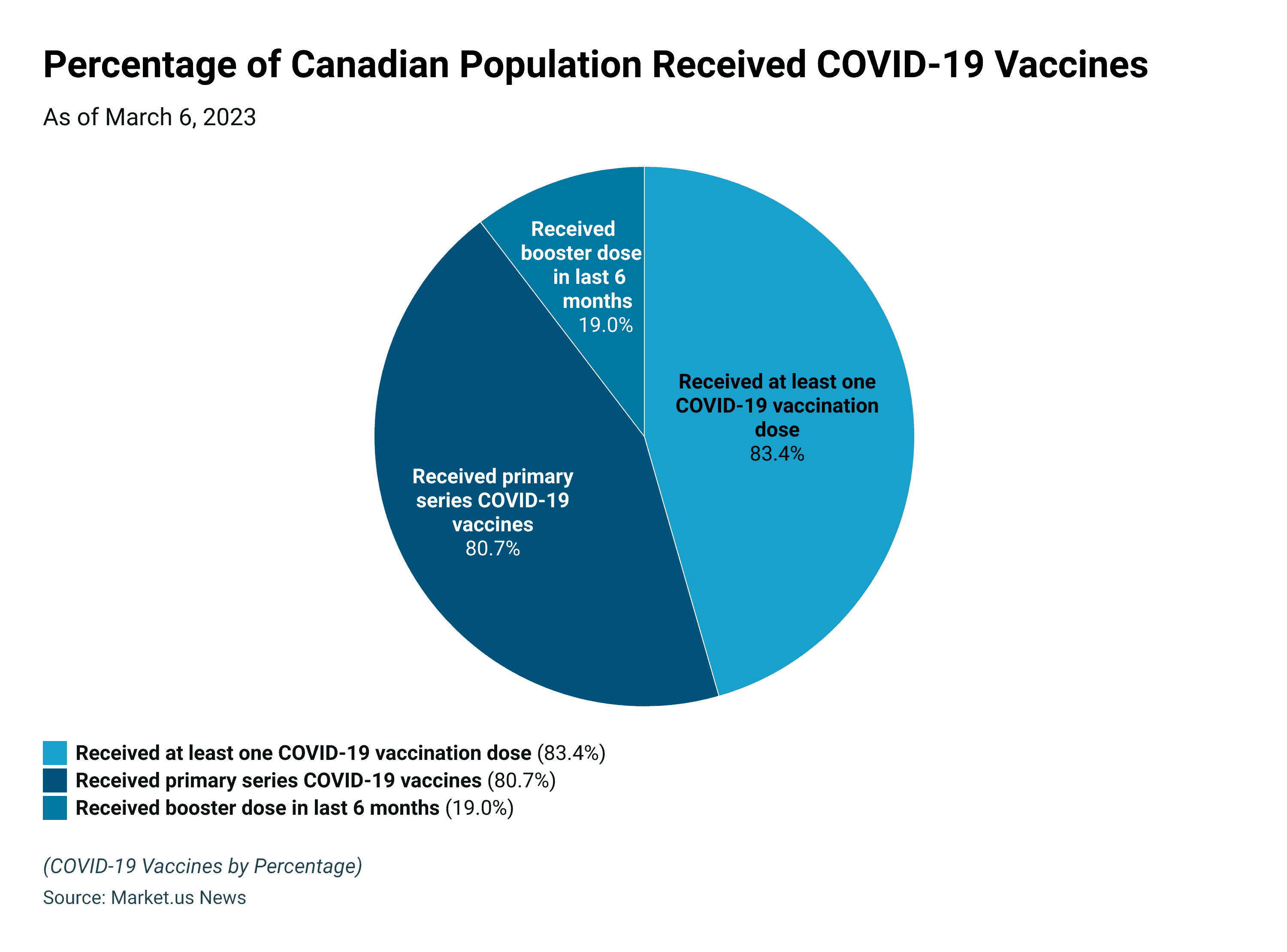
European Union
- The Pfizer-BioNTech vaccine was the first mRNA vaccine to receive authorization for emergency use in the EU.
- It was granted conditional marketing authorization by the European Medicines Agency (EMA) in December 2020.
- Subsequently, the Moderna vaccine also received conditional marketing authorization from the EMA in January 2021.
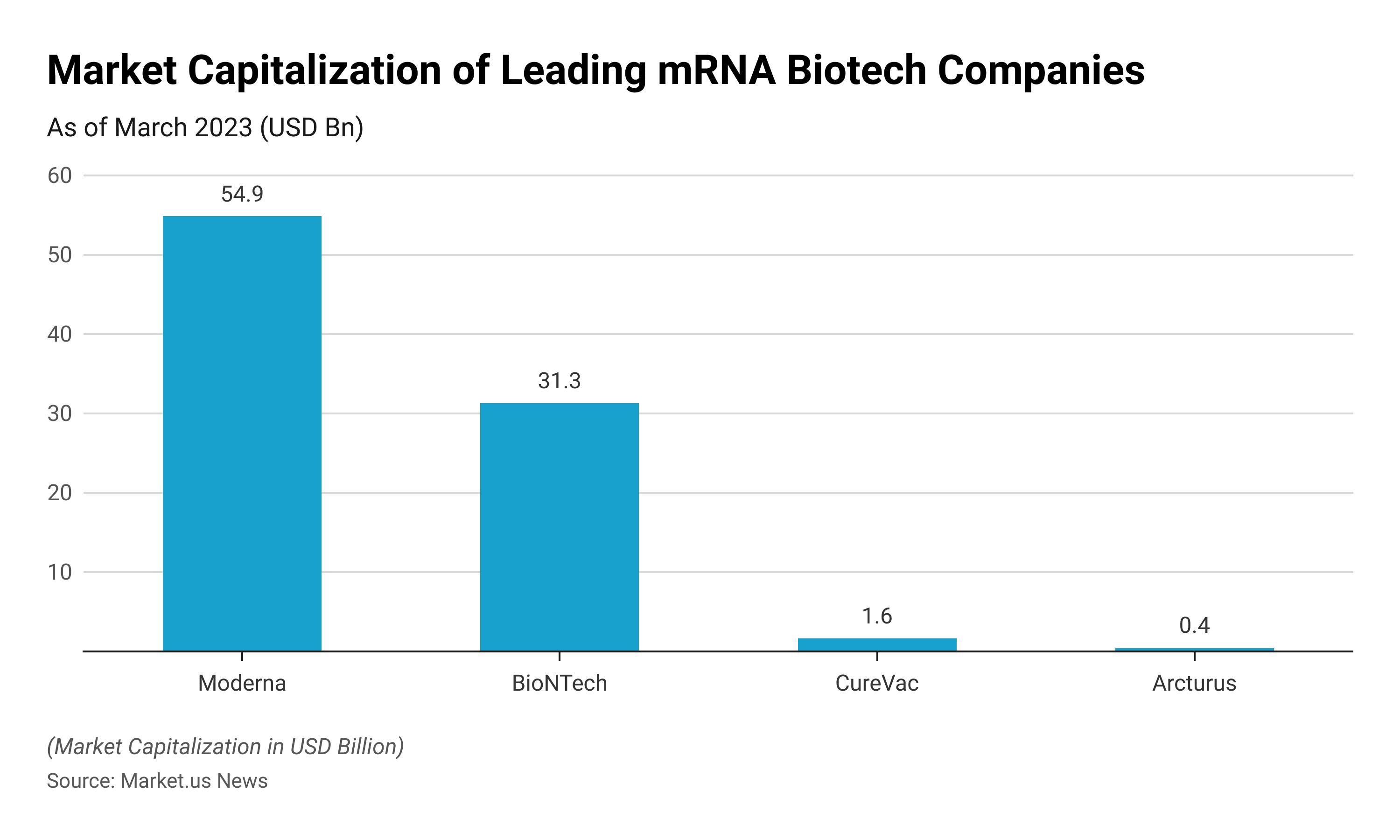
mRNA Statistics – Leading Biotech Companies (March 2023)
As of March 2023, Moderna, BioNTech, CureVac, and Arcturus Therapeutics were the leading mRNA biotech companies across the globe.
- The mRNA specialist Moderna had a market capitalization of around USD 54.89 billion as of March 9, 2023.
- BioNTech was the second leading mRNA company with a market capitalization of USD 31.27 billion as of March 2023.
- As of March 2023, CureVac and Arcturus Therapeutics have a market capitalization of USD 1.62 billion and 0.43 billion respectively.
(Source: Statista)
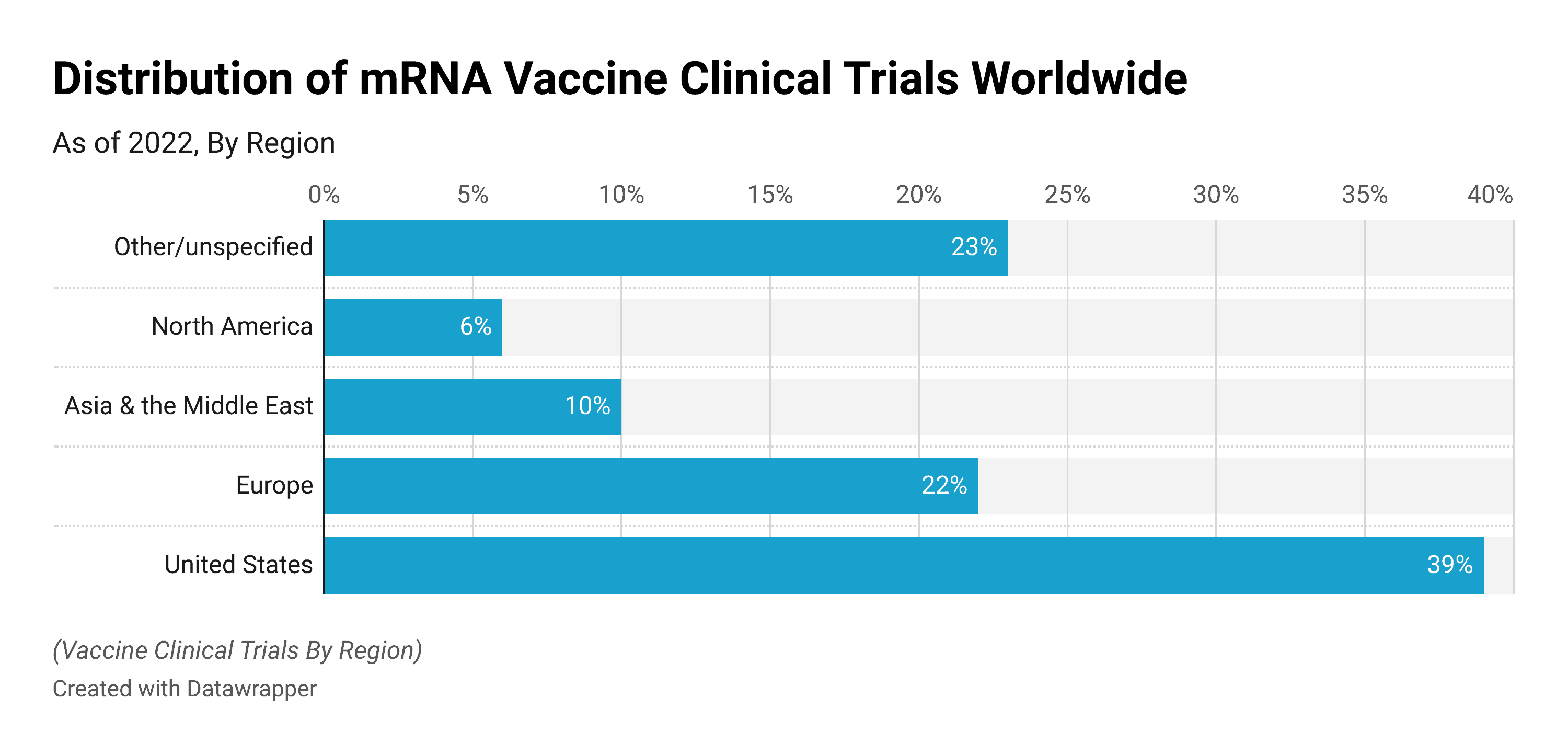
Distribution of mRNA Vaccine Clinical Trials Worldwide in 2022, by Region
- As of August 2022, about 39% of mRNA clinical trials worldwide were in the United States.
- In the same year, around 22% of mRNA clinical trials were in Europe.
- 10% of mRNA clinical trials were in Asia & the Middle East.
- Around 6% of mRNA clinical trials and 23% of mRNA clinical trials were in North America and Other countries were done.
(Source: Statista)
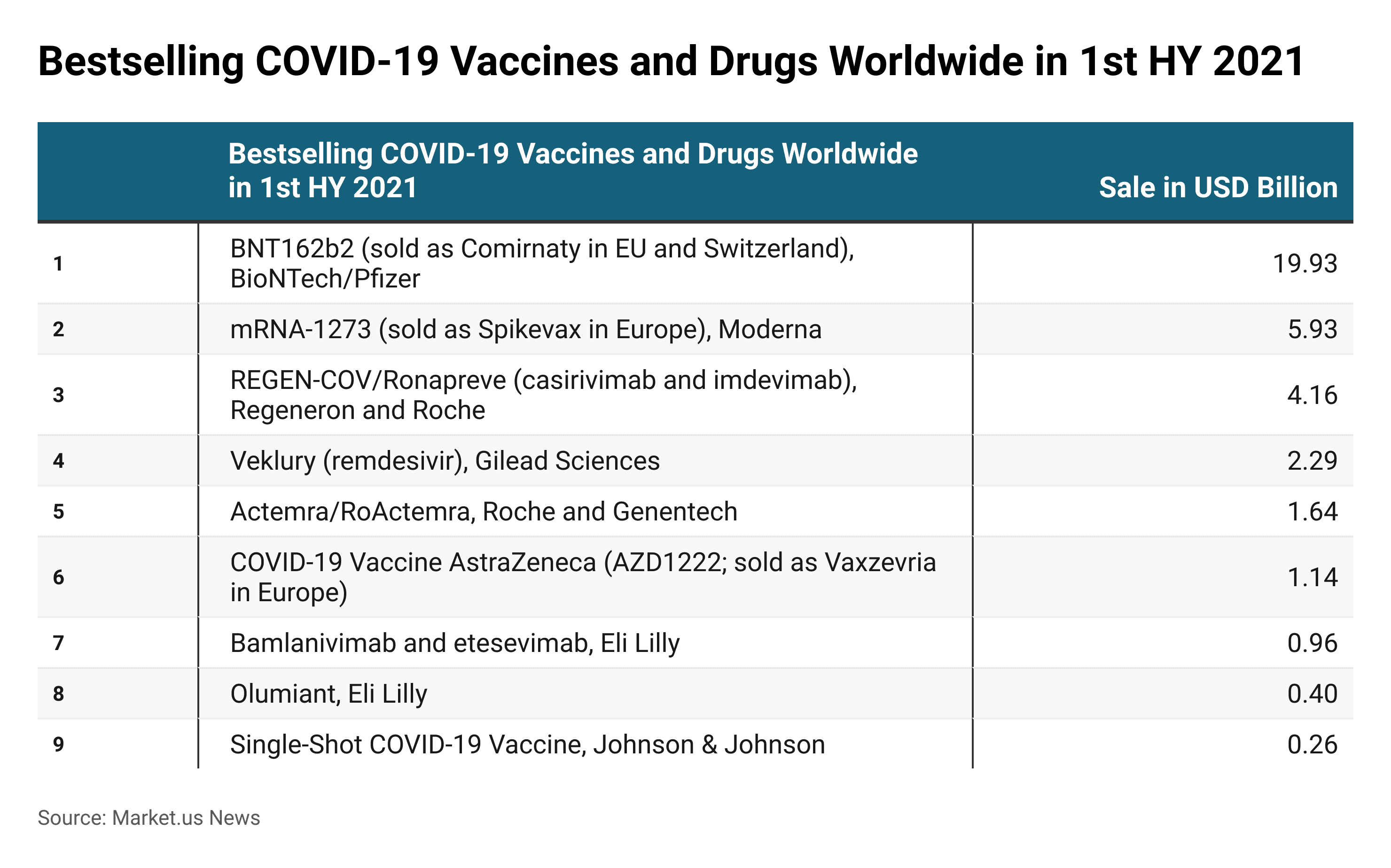
COVID-19 Vaccines and Drugs Worldwide in 1st HY 2021
- In 2021, COVID-19 vaccine Comirnaty was the top-selling COVID-19 vaccine, which generated nearly USD 19.93 billion in sales in the first half of the year 2021.
- mRNA- 1273 which is sold as a Spikevax in Europe (Moderna) was the second most selling vaccine for COVID-19, which generated global sales of USD 5.93 billion in the first half of the year 2021.
(Source: Statista)
This statistic illustrates the bestselling COVID-19 vaccines and drugs worldwide in 1st HY 2021.
Recent Developments
Acquisitions and Mergers:
- BioPharma Inc. acquired mRNA Therapeutics for $500 million, expanding its capabilities in mRNA-based drug development and therapeutic applications.
- GeneTech Group merged with mRNA Innovations, forming a strategic partnership to advance research and development efforts in mRNA technologies, with combined resources facilitating breakthrough discoveries in gene expression modulation.
New Product Launches:
- CureBio launched a novel mRNA vaccine targeting infectious diseases, demonstrating promising results in preclinical trials with a 90% efficacy rate against targeted pathogens.
- Therapix unveiled an mRNA-based therapy for cancer treatment, achieving significant tumor regression in clinical studies, with a 70% response rate observed in patients.
Funding Rounds:
- mRNABio received $100 million in Series B funding led by Biotech Investors to accelerate the development of mRNA therapeutics for rare genetic disorders, aiming for clinical trial initiation within the next year.
- ModernaGen secured $50 million in venture capital funding from Healthcare Investment Group to expand their mRNA vaccine manufacturing capacity and invest in research initiatives for novel vaccine candidates targeting emerging infectious diseases, aiming for market entry within three years.
Consumer Trends:
- Rising interest in personalized medicine and targeted therapies fueled the demand for mRNA-based treatments, with sales of mRNA therapeutics increasing by 70% compared to the previous year.
- mRNA vaccine adoption surged during the COVID-19 pandemic, with mRNA-based COVID-19 vaccines demonstrating superior efficacy and safety profiles compared to traditional vaccine platforms.
Regulatory Landscape:
- Regulatory agencies expedited the approval process for mRNA therapeutics and vaccines, implementing fast-track pathways and emergency use authorizations to address urgent healthcare needs and public health emergencies.
Wrap Up
mRNA Statistics – mRNA has gained significant attention and importance in recent years, particularly with the development of mRNA vaccines.
The top mRNA vaccines such as Moderna COVID-19 (mRNA-1273) and the Pfizer-BioNTech vaccine, BNT162b2 gained more popularity during the COVID-19 pandemic especially in the United States, Canada, and the European Union due to their higher efficacy and low side effects.
Since, COVID-19 numerous mRNA start-ups have been founded, entering. The potential applications of mRNA technology extend beyond vaccines and offer exciting possibilities for advancements in medical science.
Continued research and innovation in this area are expected to drive further breakthroughs and improvements in healthcare.
FAQs
mRNA (messenger RNA) is a type of RNA molecule that plays an important role in protein synthesis. mRNA carries genetic information from DNA in the nucleus of the cell to the ribosomes, which are the cellular structures responsible for protein production.
mRNA vaccines have undergone extensive testing and are safe and effective. They have gone through rigorous clinical trials before receiving regulatory approval. Millions of people have received mRNA vaccines, such as the COVID-19 vaccines, with a high safety profile.
Ongoing research is focused on improving mRNA technology, including enhancing mRNA stability, developing more efficient delivery systems, and expanding its applications in various medical fields. Continued innovation is expected to lead to further advancements in mRNA-based therapies.
Discuss your needs with our analyst
Please share your requirements with more details so our analyst can check if they can solve your problem(s)



